For Christopher Zimmerman, it was oysters: After a bout of nausea on a beach vacation, he could hardly touch the mollusks for months. For others, that gut-lurching trigger is white chocolate, margaritas or spicy cinnamon candy. Whatever the taste, most people know the feeling of not being able to stomach a food after it has caused—or seemed to cause—illness.
That response helps us learn which foods are safe, making it essential for survival. But how the brain links an unpleasant gastric event to food consumed hours prior has long posed a mystery, says Zimmerman, who is a postdoctoral fellow in Ilana Witten’s lab at Princeton University.
The time scale for this sort of conditioned food aversion is an order of magnitude different from other types of learning, which involve delays of only a few seconds, says Peter Dayan, director of computational neuroscience at the Max Planck Institute for Biological Cybernetics, who was not involved in the work. “You need to have something that bridges that gap in time” between eating and feeling ill, he says.
A newly identified neuronal circuit can do just that. Neurons in the mouse brainstem that respond to drug-induced nausea reactivate a specific subset of cells in the animals’ central amygdala that encode information about a recently tasted food. And that reactivation happens with novel—but not familiar—flavors, according to work that Zimmerman presented at the annual COSYNE meeting in Lisbon last month.
With new flavors, animals seem primed to recall a recent meal if they get sick, Zimmerman says. As he put it in his talk, “it suggests that the common phrase we associate with unexpected nausea, that ‘it must be something I ate,’ is literally built into the brain in the form of this evolutionarily hard-wired prior.”
M
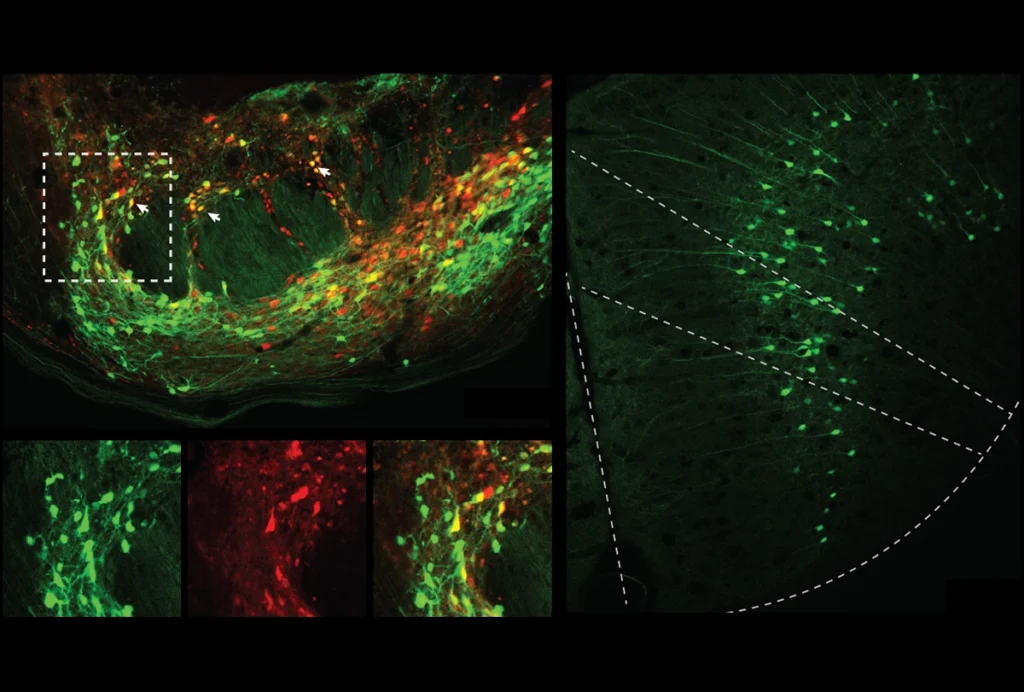
ice in the experiment developed a strong aversion to a novel flavor of Kool-Aid when treated with an emetic drug 30 minutes after drinking it, the team found: When the animals were later given a choice between the Kool-Aid or water, they preferred to drink water. Mice already familiar with that flavor of Kool-Aid, however, did not develop the same aversion, even though they showed the same signs of malaise after the drug treatment.Novel flavors, it turns out, evoke a different pattern of neuronal activation than familiar flavors do, the team discovered when they measured the animals’ brain-wide levels of FOS—a protein whose expression increases with neuronal activity. In mice tasting a flavor for the first time, activity spiked in the sensory areas and in the amygdala, which was also activated when the animals exhibited malaise-like behaviors. But when the flavor was familiar, different brain areas—such as the lateral septum and ventral hippocampus—showed the most activity.
Neurons in the central amygdala activate after mice consume a new flavor of food, the team found using Neuropixels probes. The cells quieted down soon after, but they reactivated when the team later induced “virtual malaise” by optogenetically stimulating calcitonin gene-related protein (CGRP) neurons in the brainstem, which are known to contribute to feelings of illness. That reactivation did not happen for neurons that responded to familiar flavors—suggesting that the novel-flavor cells have a heightened intrinsic excitability, Zimmerman says. The team initially posted the study as a preprint on bioRxiv in October and updated it in January.
Cells that are reactivated by the stimulation of CGRP cells ultimately develop stronger flavor representations in the amygdala, the team found by decoding the neuronal activity in response to different flavors.
“It’s this built-in novelty-detection circuitry, which compares a flavor to any flavor you ever had before,” Witten says. If the flavor is novel, she says, neurons that encode it are ready to be hardwired for reactivation by the CGRP cells. That highlights how evolutionary pressures can shape this form of learning, she says: The brain evolved expectations for which associations should be learned in a given environment—and novel flavors are often important signals.
When a new flavor does not give rise to feelings of illness, on the other hand, that neuronal representation becomes less distinct over time, the team found. Eventually, the response to a familiar flavor in the central amygdala is indistinguishable from the response to water; the flavor is considered safe, Zimmerman says.
I
t remains unclear whether the findings will translate to primates. Some primate studies, for example, have suggested that malaise-responsive cells project to the thalamus and the insula before sending signals to the amygdala.Another looming question is how the malaise cells specifically reactivate the relevant novel-flavor cells in the amygdala, says Timothy Behrens, professor of computational neuroscience at the University of Oxford, who was not involved in the work. The novel-flavor cells are not active for long enough to bridge the 30-minute delay the mice experienced between tasting a new flavor and having an unpleasant gastric reaction, so some other signal must be involved in making the connection between the experiences, he says.
The team suspects that a biochemical tag prepares the novel-flavor cells for reactivation, much in the way that CREB activity can pull hippocampal neurons into encoding information about a particular memory, Zimmerman says. The idea, he says, is that those second messengers can persist for a long time and end up making the cells more excitable. He and his colleagues are looking into that possibility, he says.
They are also testing the causal relationship between the CGRP cells’ reactivation of the novel-flavor cells and aversion to that flavor, Zimmerman says.
Moving forward, it would be interesting to examine whether similar reactivation mechanisms are at play in the learning of positive food associations, says Ivan de Araujo, director of body-brain cybernetics at the Max Planck Institute for Biological Cybernetics, who was not involved in the new work. “Certainly, the CGRP neurons are not the answer [in that case]. But then the question is whether this general arrangement they are proposing is what makes the system work.”