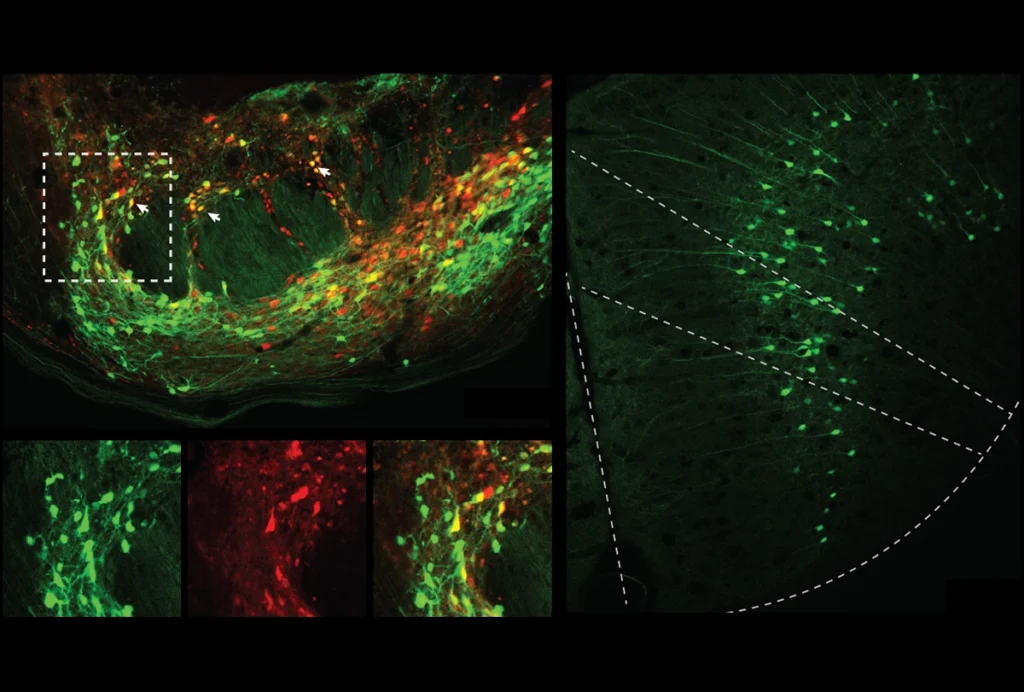
Dozens of green and red magnets littered the three whiteboards in the meeting room. Each one signified a vote — a yea or nay on various claims about a puzzling neural phenomenon called “representational drift.” Put simply, representational drift describes the observation that, over time, a group of neurons can change their response to some stimulus, despite no apparent changes in the animal’s perception or behavior.
As the scattered magnets suggested, there was little consensus among the roughly 100 neuroscientists in the room, who gathered this past April for a four-day meeting at the Janelia Research Campus in Ashburn, Virginia.
“In some people’s eyes, [representational drift] is sort of a controversial idea,” says Timothy O’Leary, professor of information engineering and neuroscience at the University of Cambridge in the United Kingdom, who helped to organize the meeting and recounted the whiteboard poll.
That’s in part because representational drift defies expectations that stable behaviors should derive from stable neuronal activity. So much so that when several independent teams first identified it nearly two decades ago, many dismissed it as experimental error: Perhaps the electrodes recording the neuronal activity had slipped over time and started recording different cells, for example, or the data had been analyzed improperly.
In recent years, however, other researchers have confirmed some of those early findings — thanks to technology advancements that help experimentalists track the responses of single neurons over the course of many months. The new findings spurred O’Leary and his co-organizers to plan the standalone April meeting. From O’Leary’s vantage, representational drift is akin to how a nation’s foreign policy can remain stable across decades, even as the elected individuals who shape that policy enter and leave office.
The vote at the April meeting came before the talks began, and attendees proffered a range of possible mechanistic explanations, which the organizers wrote on the whiteboard — is representational drift an “unavoidable side effect of ongoing learning,” as some suggested, or a “way of encoding information,” as others voted? A minority was skeptical in general, with five attendees voting that drift is either “largely a measurement artifact” or “a result of low statistical power/poor analysis.” (“I don’t want to believe that, but I do,” responded one attendee, as memorialized in a photograph of the whiteboard.)
But by the time the meeting was over, the number who definitively saw drift as an artifact or analysis problem had dropped to zero. The evidence for drift being a fundamental property of the brain is mounting, O’Leary says. And despite the lingering doubts, the meeting signaled a shift in how neuroscientists are thinking about representational drift.
“The bottom line is that the data now forces people to confront this phenomenon,” says meeting co-organizer Andrew Fink, a postdoctoral researcher in Richard Axel’s lab at Columbia University. “It’s hard to argue that it doesn’t exist. And so now we’re tasked with thinking about what it means.”
A
s researchers probe the brain for signs of representational drift, a pattern has started to emerge: Some brain areas seem to exhibit more drift than others.Place cells in the CA1 region of the hippocampus, for example, exhibit significant drift, as do neurons in the posterior parietal cortex, two mouse studies suggest. Even after the mice learned to navigate their environment or perform a particular task, the associated neuronal activity in either brain area changed over time. Learning seemed to have stopped, yet the animals’ brain responses continued to change.
The piriform cortex, which is involved in olfaction, is another hub for representational drift, according to a 2021 mouse study. Electrodes implanted in that brain region stably recorded neuronal activity as the animals were exposed to odors over 32 days. Looking at the activity of all recorded cells, the researchers could identify which odor the animal was smelling, but the individual cells that made up that response changed over the course of the study.
“Our interpretation of that data is that it reflects some ongoing learning process,” even after the animals had seemingly become familiar with an odor, says Fink, lead researcher on the study.
Not all brain areas need to participate in such flexible learning, and so, the theory goes, not all exhibit the same amount of drift. Drift in the auditory, visual or somatosensory cortex seems to happen on a smaller scale than it does in the hippocampus or posterior parietal cortex, for example, O’Leary says. Cells in the rat motor cortex remain stable over long periods of time, according to 2022 findings. And neurons that respond to faces show hardly any drift at all, retaining the same responses for up to a year, according to a 2014 study in monkeys.
The baseline amount of drift across various regions seems to provide a measurable signal of plasticity at the level of a neural population, O’Leary says. And studying it “gives you a quantitative window into what’s happening in different parts of the brain.”
How much randomness, or noise, is present in the neural signal may account for these differing amounts of drift, although the mechanisms remain unclear.
According to one hypothesis, “drift is a byproduct of noisy learning” — meaning that neurons continue to respond to the randomness within an animal’s environment, says Farhad Pashakhanloo, a postdoctoral fellow at Cold Spring Harbor Laboratory in New York. In a presentation at the Society for Neuroscience meeting last month, Pashakhanloo and his colleagues proposed a model in which the randomness in the environment leads to a certain amount of randomness in neuronal responses, and that, in turn, nudges the cells to drift together in a way that maintains stable task performance, he says.
Some parts of the brain, such as the retina or the motor system, may have had evolutionary pressure to permit less randomness in their neuronal responses, leading to less drift, Fink says. “In areas where there is less of a constraint on stability, maybe that noise is more tolerated.”