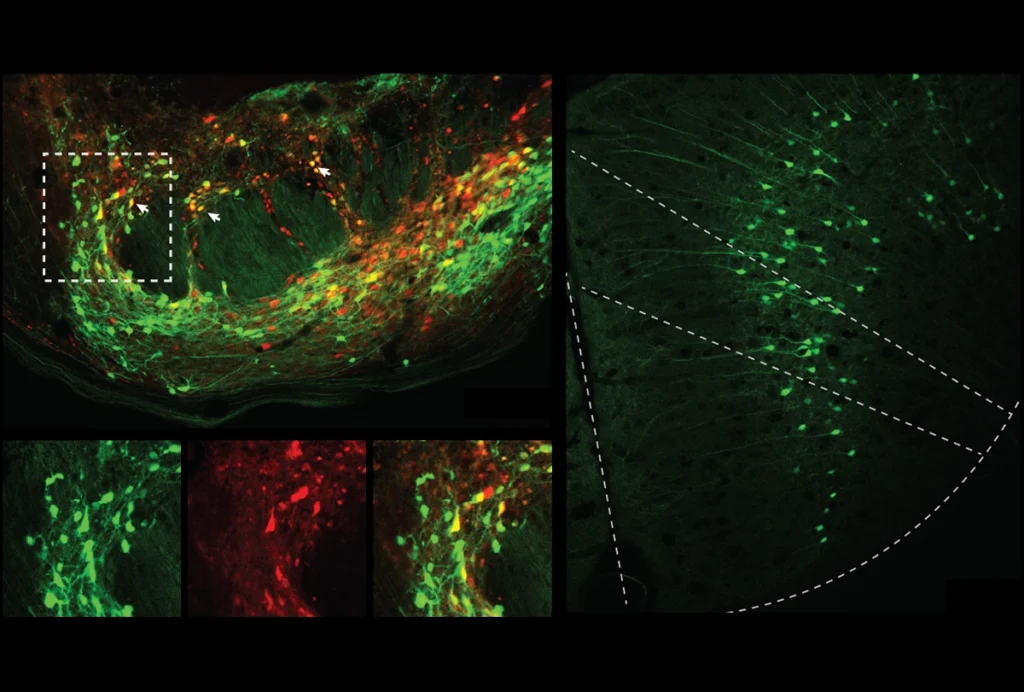
For two years, Veronica Egger couldn’t figure out the electrical oscillations she had been measuring in the olfactory bulb.
Egger, professor of neurophysiology at the University of Regensburg, was using a semi-intact preparation, in which a rat’s brain was accessible for recordings but did not receive input from the brainstem or the rest of the body.
A peristaltic pump pushed artificial blood into the brain’s vasculature in a steady rhythm. An electrode in the olfactory bulb detected electrical oscillations that followed each cycle of the pump, entrained on its frequency.
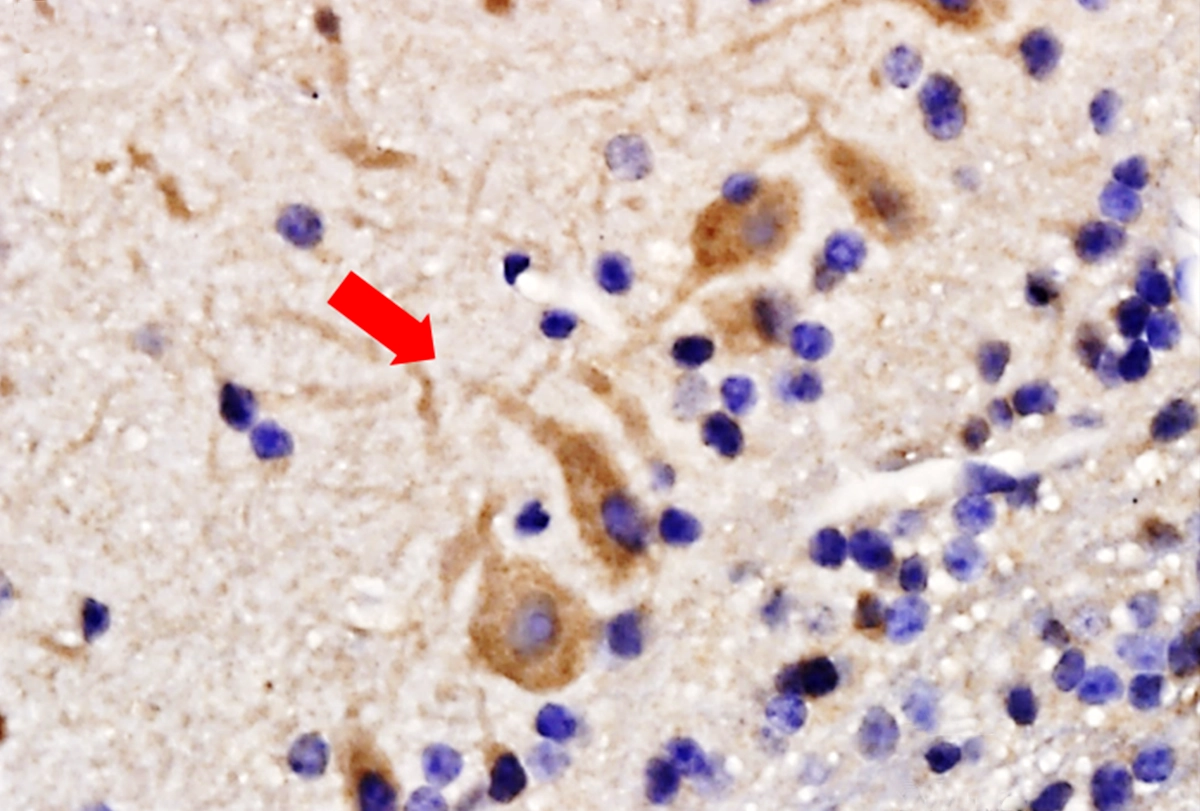
Network oscillations are a hallmark of olfactory bulb activity, Egger says: Mitral cells, the primary neuron type in the region, fire in sync with respiration, and other oscillations organize faster bursts of activity. But in this preparation, the brain did not receive input from the lungs, and no airflow came in through the nose; the neurons couldn’t be firing in sync with respiration, because there wasn’t any.
“We went back and forth, and there was always some evidence for artifact, and there was evidence for biology, and it was super puzzling,” Egger says. “And then somehow, after two years, I had this kind of intuition.”
What if, she recalls wondering, the neurons were responding to the pressure changes in the vasculature? Mitral cells in the mouse olfactory bulb contain Piezo2 receptors, which detect mechanical signals, a lab in Texas reported in 2021. And the pump Egger was using produces pulsations in the brain’s vasculature that are similar in amplitude and frequency to the pressure changes caused by the heart—a “big serendipity,” Egger says.
Through a series of experiments in the semi-intact preparation and in awake animals, Egger and her colleagues demonstrated that the oscillations were generated by mitral cells that synchronized with the heartbeat by directly detecting blood pressure changes. The resulting paper was published in Science on 2 February.
“In the end, it was big fun, but in between, it was quite nerve-racking,” Egger says. “It’s more like a true-crime story.”
The results provide a new, more direct mechanism for how the brain receives information about the heartbeat, says Catherine Tallon-Baudry, a CNRS senior researcher in cognitive neuroscience at École Normale Supérieure.
T
he oscillations Egger measured were not due to the electrode picking up stray electrical activity in the environment, because they stopped when she added an extra reservoir to the pump that decreased the pressure it produced to 25 percent. The oscillations also disappeared when she switched the pump’s fluid to a nonoxygenated artificial blood, which indicates they were produced by cells.They also vanished when the team blocked Piezo1 and Piezo2 mechanoreceptors with tarantula venom, which left regular spiking intact, but not when the researchers blocked sodium channel activity with lidocaine.
Egger and her team did not do genetic knockout or antibody experiments to determine which mechanical receptor was responsible for the oscillations, but they could zero in on Piezo2 based on the characteristics of the oscillation’s waveform, says Owen Hamill, associate professor of neurobiology at the University of Texas Medical Branch, who discovered Piezo2 receptors in the mouse olfactory bulb. Hamill wrote a commentary for Science about Egger’s paper but was not involved in her study.
“What they were able to model is that it was only the Piezo2 gating that could explain the waveform of the heartbeat-related oscillation,” he says. “That was the exciting thing for me.”
The rhythm of the pressure changes also synchronized spontaneous spiking in the mitral cells. These experiments indicate that the oscillations are caused by direct mechanical detection in neurons rather than through synaptic transmission. “That’s a new type of oscillation, because all the others were mediated by synapses. This is mediated by a mechanical event in the brain,” Hamill says. “This is potentially a paradigm shift, if the finding is confirmed in humans.”
But “it’s not definitive proof that the mitral cells are sensing the pulsation,” says Igor Felippe, a research fellow in Julian Paton’s lab at the University of Auckland. The brain physically pulses from blood flow and can bump into the electrode, he says, which could be detected by mechanoreceptors. Replicating the experiments with a technique such as calcium imaging could verify whether the neurons are indeed firing from the pressure pulsations and not from physical contact with the electrode, he adds.
About 15 percent of the neurons in the olfactory bulb of awake, head-fixed mice were entrained by the heartbeat, the group found. “The neurons can basically feel the pulse within the brain,” Egger says. “There is a direct way within the brain to know about the heartbeat.”
I
t has become more apparent in recent years that the brain keeps tabs on internal body signals, even in areas that are not traditionally thought to have an interoceptive role, Tallon-Baudry says.She says she often hears from colleagues about strange, noisy data that suggested body signals are influencing neural activity, “but they considered that it was probably some kind of artifact and never published it,” she says. Now that Egger and her team have teased out this new mechanism, “I’m sure there will be data from other labs in other regions, very quickly after this paper,” Tallon-Baudry says. “I think this paper will be a fundamental game changer.”
But the purpose of mechanical detection in neurons is still speculative, Tallon-Baudry says. One possible explanation is that entraining on the heartbeat could be a way to synchronize activity in far-apart brain regions. If distant neurons fire in response to the same stimulus, more blood will be directed toward them, which could cause the neurons to start firing in sync with the pressure changes and thus each other.
It could also be a way for the brain to more quickly receive information about the heartbeat, says Sahib Khalsa, director of clinical operations at the Laureate Institute for Brain Research and assistant professor of community medicine at the University of Tulsa. Direct detection in neurons is faster than receiving synaptic messages that must travel from the heart to the vagus nerve and then through several brain regions, he says.
Egger says it would be helpful to replicate the experiments in awake, behaving animals, but that is outside of her expertise. “Others need to figure out what’s really going on,” she says, “and if we can contribute a bit more to the biophysics of this coupling, I would be really happy to look into that.”