Cortical interneurons derive differently in human brains
Excitatory neurons and some inhibitory neurons in the adult human cortex share parents, challenging the longstanding idea that the two cell types have different origins.
Two new postmortem tissue studies have bolstered an old idea about the origins of interneurons in the human cerebral cortex, providing evidence that both excitatory and some inhibitory neurons there descend from the same parent cells.
The results, from independent teams, challenge the longstanding belief — based largely on research in mice — that each class of cortical neurons has a distinct provenance: Inhibitory neurons form in the ventral telencephalon (which gives rise to subcortical structures) and then migrate to the cortex, where excitatory neurons are born.
“We’re not saying the mouse work is wrong; we’re just saying the models for how the human brain is assembled need to be updated,” says Joseph Gleeson, professor of neuroscience at University of California, San Diego, who led the work described in one of the preprints, which was posted on bioRxiv in October.
Scientists first proposed about three decades ago that human excitatory and inhibitory neurons have shared origins, but the idea remained controversial because early studies yielded conflicting results.
“Myself and many other people kind of pooh-poohed them — but they were right all along,” says Christopher Walsh, chief of genetics and genomics at Boston Children’s Hospital in Massachusetts and lead investigator on the analysis described in the other preprint, which his team posted on bioRxiv in November.
Both Gleeson and Walsh used genetic sequencing to trace the family trees of single neurons from human postmortem brain tissue. The results provide the first direct evidence of the cells’ shared origins in people and align with other recent research showing that human stem cells can generate both excitatory and inhibitory neurons in in-vitro experiments.
This confirmation is important for understanding neurological conditions that involve these two cell populations, Walsh says. It may help to explain why epilepsy in adults nearly always occurs in the temporal lobe, for example, or how autism or schizophrenia arise.
S
tem cells can acquire genetic variations as they divide during development. Daughter cells with these so-called somatic mosaic variants stand out against their otherwise identical neighbors, like different-colored tiles in a mosaic.Because mature neurons do not continue to divide, their genetic variants provide a clear signature of their ancestry. The earlier in development a variant occurs, the more likely it is to be shared by a large fraction of cells. If fewer cells carry a variant, it has cropped up more recently, in later-diverging cells.
Both of the new studies used sensitive sequencing methods to pick out somatic mosaic variants in individual neurons but focused on different brain regions. Gleeson’s team compared the somatic mosaic variants in excitatory and inhibitory neurons taken from the cortex, basal ganglia, hippocampus, thalamus and cerebellum of two unrelated neurotypical adults. Walsh’s team analyzed overlapping variants in neurons from the prefrontal cortex, the primary visual cortex and the secondary visual cortex in two other unrelated neurotypical adults.
Some cortical inhibitory and excitatory neurons from each donor shared anywhere from roughly a dozen to three dozen variants, the two studies found. About 2 percent or less of all cells shared these variants, Walsh’s work shows, indicating that the variants appeared late in development.
Although the sample size is small, “it doesn’t take much data to show that something is present,” Walsh says. “In theory, you’d only need like one or two [variants], and we’ve got a dozen or more.”
Taken together, the work provides “strong evidence” that human cortical stem cells can create both excitatory and inhibitory neurons, says Guo-li Ming, professor of neuroscience at the University of Pennsylvania in Philadelphia, who was not involved in either study. “The stem cells are more versatile — they’re not as restricted as you would think.”
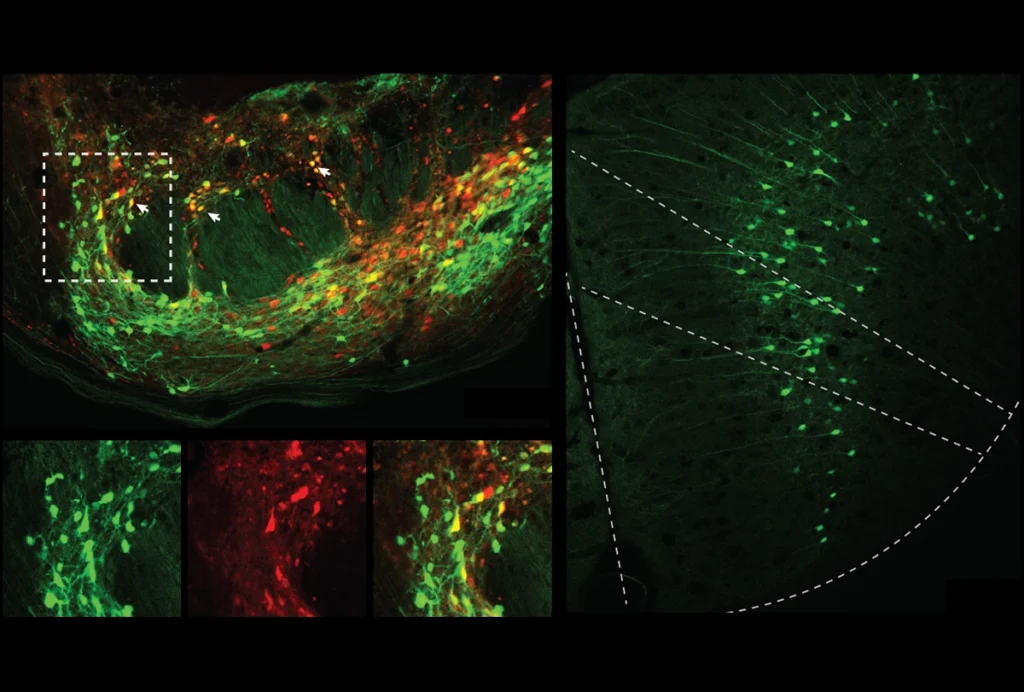
Some human inhibitory neurons do still migrate to the cortex from the basal ganglia, as seen in mice, Gleeson’s study shows. These neurons derive from the caudal ganglion eminence — an early basal ganglia structure — but lack the variants shared by cortically born inhibitory and excitatory neurons.
The migratory interneurons may represent the majority, Walsh’s work indicates. Past studies of the human brain found a 7-to-3 ratio of excitatory to inhibitory neurons. But only a 10-to-1 ratio share the same rare variants, his team found, suggesting that a minority of inhibitory neurons begin in the cortex.
The variants also made it possible to track how cells disperse during development. For example, few of the variants found in cells from the hippocampus also appear in cells in the cortex or basal ganglia, which means the hippocampal cells are likely born there and stay put, Gleeson’s work shows. Similarly, the visual cortex harbors cells with more unique variants than do other cortical areas Walsh’s team sampled.
But variants found in cells from the frontal cortex crop up across the cortex, according to Walsh’s study. And not only do excitatory neurons disperse across the cortex, but they also disperse out from deeper cortical layers, Walsh’s analysis shows.
These dispersal results further upend the common hypothesis that cells from the same parent form into columns as generations progress, as is seen in rodent brains, Ming says. Instead, sibling cells in human brains can scatter across the cortex.
This finding in particular could complicate research on certain diseases: Pathogenic mutations can be ingrained in cells far outside the brain region most affected, Walsh’s work suggests.
B
oth preprints cast Walsh and Gleeson as detectives amid a crime scene, peering at the evidence to deduce how and when the crime took place, says Tomasz Nowakowski, associate professor of neurological surgery at University of California, San Francisco, who was not involved in either study. But their approaches, though powerful, cannot pinpoint exactly when or where the cells diverged, he says. “You haven’t been there when the crime was committed, so you cannot really tell.”It’s a forensic gap Walsh says he plans to address in the future using fetal tissue. “It stands to reason that the progenitor is a radial glial cell, but we don’t have direct proof of that,” he says.
The exact subtype of inhibitory neuron detected in Gleeson’s work appears to differ from previous in vitro experiments, says Arnold Kriegstein, professor of neurology at the University of California, San Francisco, who was not involved in either study. This difference might reflect the small number of cells analyzed retrospectively or a shift in markers expressed as cells age. “I think that still remains an interesting and perhaps still unresolved issue,” Kriegstein says.
Additional studies can track the development of mutations in diseases, such as amyotrophic lateral sclerosis, Walsh says. They could also look at autism, which some attribute, in part, to an imbalance between inhibitory and excitatory neurons, Gleeson says.
Recommended reading
Uncertainty and excitement surround one company’s cell therapy for epilepsy
Cerebellar circuit may convert expected pain relief into real thing
Explore more from The Transmitter
Building an autism research registry: Q&A with Tony Charman
New ‘decoder’ tool translates functional neuroimaging terms across labs