- Three overarching patterns of gene transcription involved in brain development inform how autism and schizophrenia emerge from altered scripts. Nature Neuroscience
- Mice missing the autism-linked gene SHANK3 show changes in the spatial and molecular organization of postsynaptic densities. Molecular Psychiatry
- More brain cells in female mice have active X chromosomes from their mother (60 percent) than from their father (40 percent), which results in more fragile X syndrome-like behaviors in female offspring when gene variants are inherited maternally. Cell Reports
- MECP2 gene variants that cause Rett syndrome lead to altered RNA polymerase interactions and DNA binding and, ultimately, decreased gene expression. Neuron
- Inherited factors contribute to 87 percent of autism cases in boys and 75.7 percent in girls, suggesting differing underlying causes overall. JAMA Psychiatry
- Gene variants that are associated with autism but have only moderate effects often co-occur with other moderate-effect-size genes, leading to large effects, according to a preprint. medRxiv
- People with 16p11.2 deletions are more likely to have gross-motor impairments and less likely to have fine-motor impairments than people with 16p11.2 duplications. Autism Research
- Oligodendroglia contribute to the myelination of neuronal axons in mice; but deletion of the autism-linked SCN2A gene alters this process and leads to hypersensitivity in auditory pathways, according to a preprint. bioRxiv
X chromosome inactivation; motor difficulties in 16p11.2 duplication and deletion; oligodendroglia
Here is a roundup of autism-related news and research spotted around the web for the week of 6 May.
By
Jill Adams
7 May 2024 | 2 min read
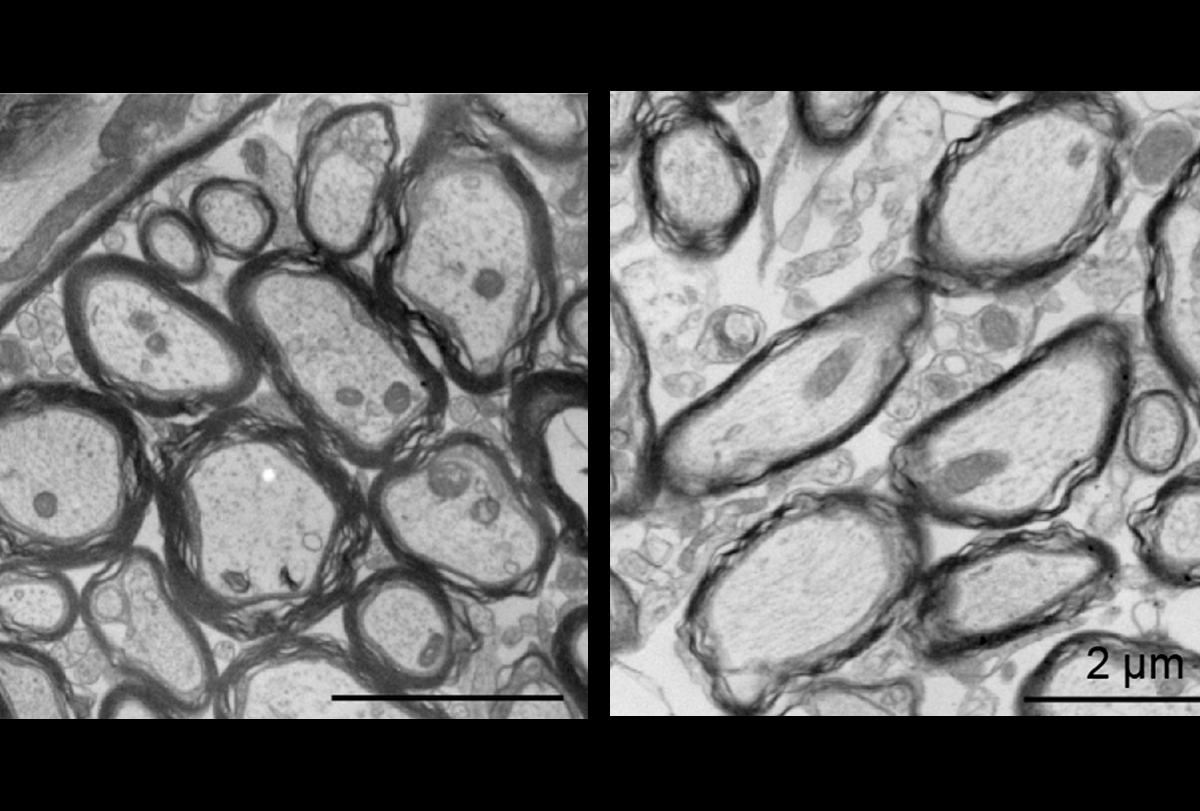
Under covered: Loss of SCN2A in oligodendroglia (right panel) leads to thinner myelin sheathing and larger axon diameter compared with wildtype mice (left panel).
- A coding system can automate measures of rate of speech and conversational turns in minimally verbal autistic children. Autism Research
- In mice missing the SHANK3 gene, restoring HCN2 channel activity early in development ameliorates the sensory and sleep problems that are typical in this animal model of autism. Cell Reports Medicine
tags:
Recommended reading
Building an autism research registry: Q&A with Tony Charman
By
Cathleen O’Grady
25 July 2024 | 8 min read
CNTNAP2 variants; trait trajectories; sensory reactivity
By
Jill Adams
23 July 2024 | 2 min read
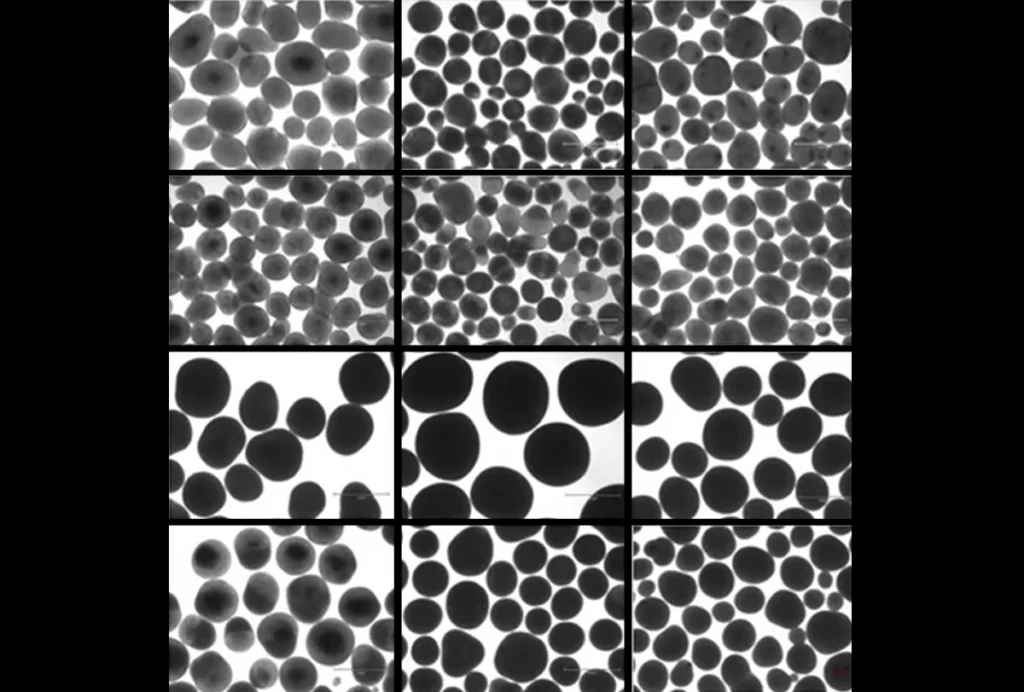
Brain organoid size matches intensity of social problems in autistic people
By
Holly Barker
18 July 2024 | 5 min read
Explore more from The Transmitter
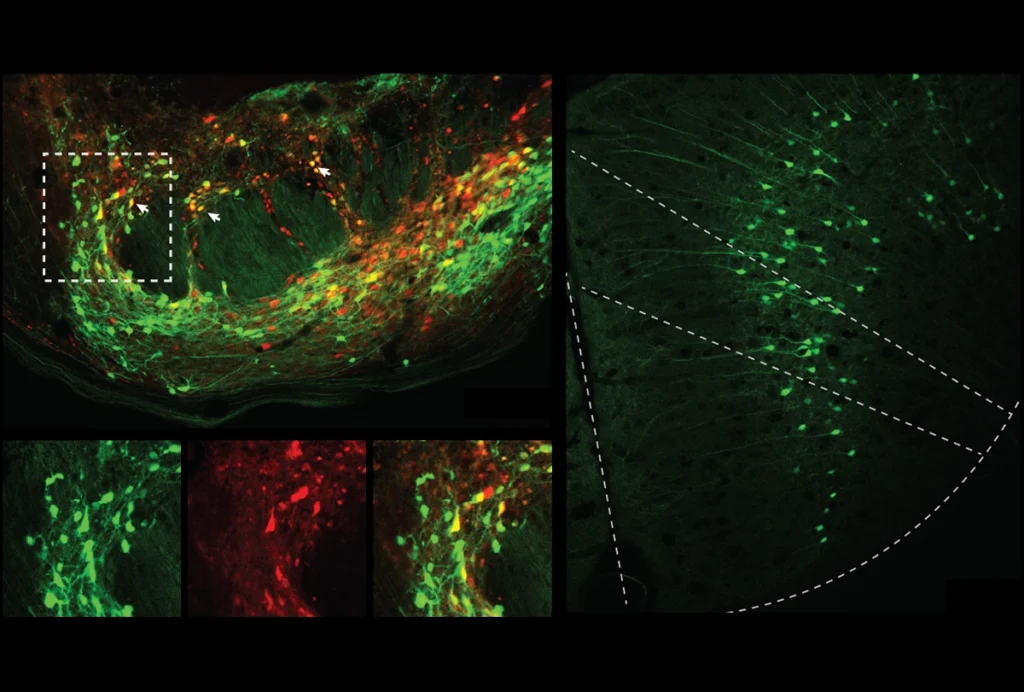
Cerebellar circuit may convert expected pain relief into real thing
By
Angie Voyles Askham
24 July 2024 | 6 min read
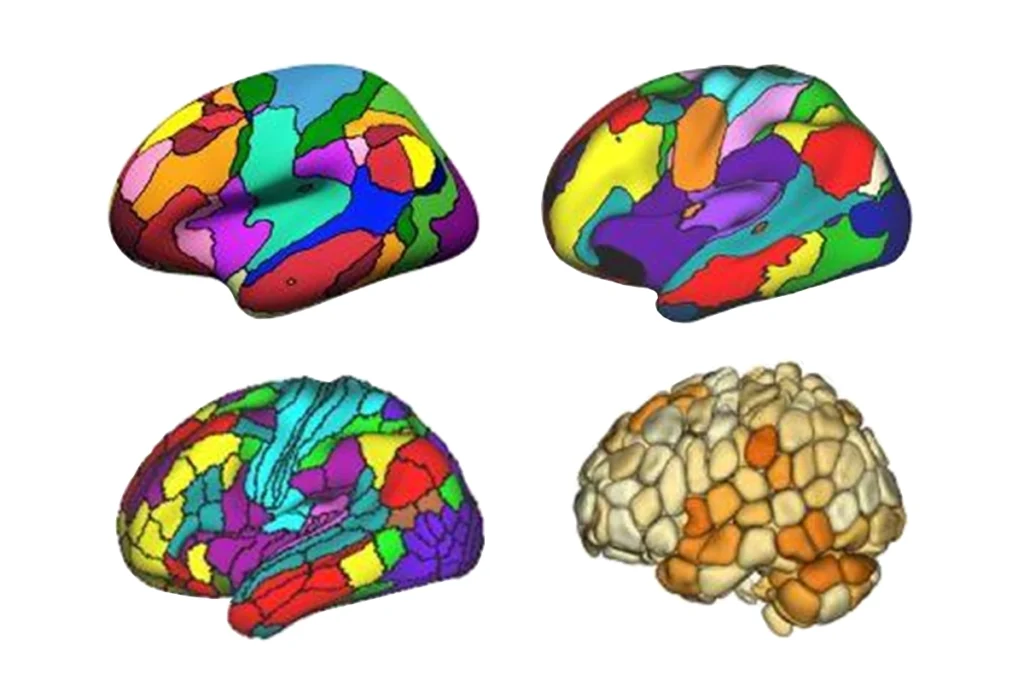
New ‘decoder’ tool translates functional neuroimaging terms across labs
By
Holly Barker
23 July 2024 | 4 min read
Cite this article: