Our understanding of memory is often summed up by a well-worn mantra: Neurons that fire together wire together. Put another way, when two brain cells simultaneously send out an impulse, their synapses strengthen, whereas connections between less active neurons slowly diminish.
But there may be more to it, a new preprint suggests: To consolidate memories, synapses may also influence neighboring neurons by using a previously unknown means of communication.
When synapses strengthen, they release a virus-like particle that weakens the surrounding cells’ connections, the new work shows. This novel form of plasticity may aid memory by helping some synapses to shout above the background neuronal hubbub, the researchers say.
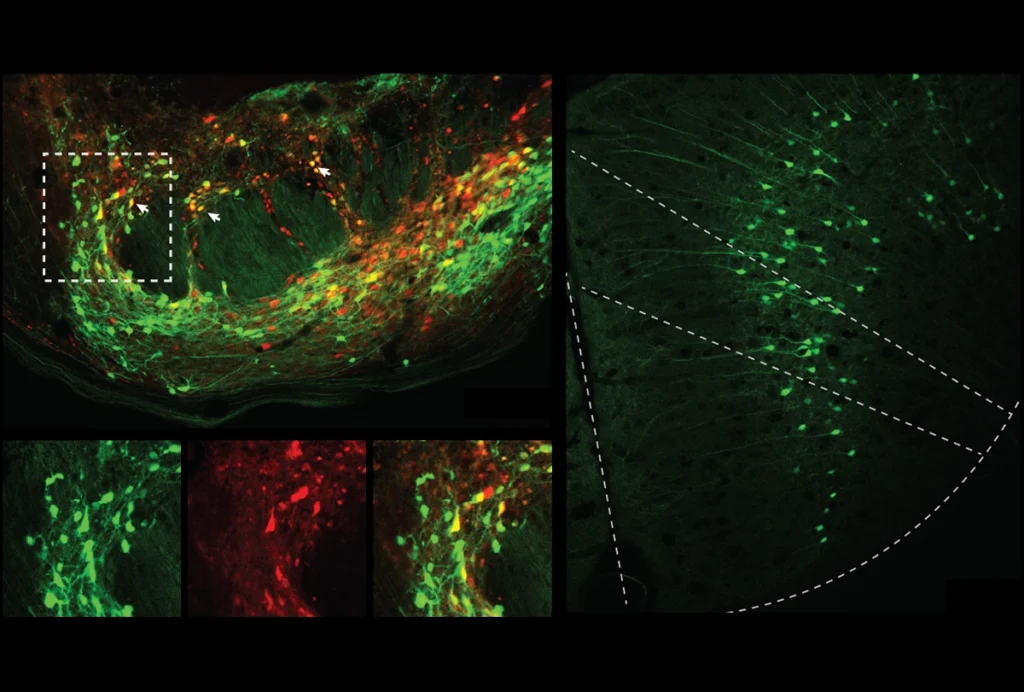
The mechanism involves the neuronal gene ARC, which is known to contribute to learning and memory and encodes a protein that assembles into virus-like capsids—protein shells that viruses use to package and spread their genetic material. ARC capsids enclose ARC messenger RNA and transfer it to nearby neurons, according to a 2018 study. This leads to an increase in ARC protein and, in turn, a decrease in the number of excitatory AMPA receptors at those cells’ synapses, the preprint shows.
“ARC has this crazy virus-like biology,” says Jason Shepherd, associate professor of neurobiology at the University of Utah, who led the 2018 study and the new work. But how ARC capsids form and eject from neurons was unclear, he says.
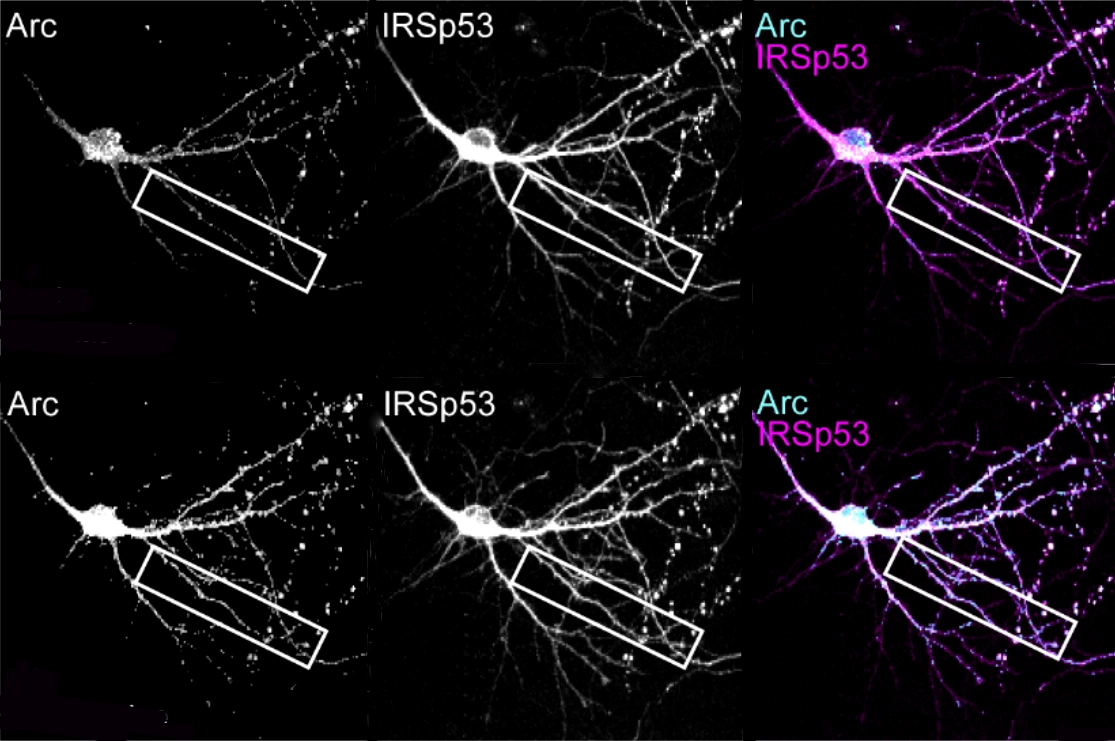
As it turns out, synaptic strengthening spurs ARC capsid release, according to the preprint. When neuronal connections strengthen, ARC capsids are packaged into vesicles, which then bubble out of neurons through their interactions with a protein called IRSp53. Surrounding cells absorb the vesicles containing ARC, which tamps down their synapses, the new work suggests.
“They have taken the story a long way,” says Clive Bramham, professor of biomedicine at the University of Bergen, who was not involved in the work. “It’s a lot of work, nicely put together.”
But the results—all from in vitro experiments—need to be replicated in animals before any firm conclusions can be drawn, Bramham adds. “This is in neuronal cultures, so we don’t really know to what extent this is going to apply in adult tissue. It may be that in cultured neurons, the release of vesicles is way more important.”
S
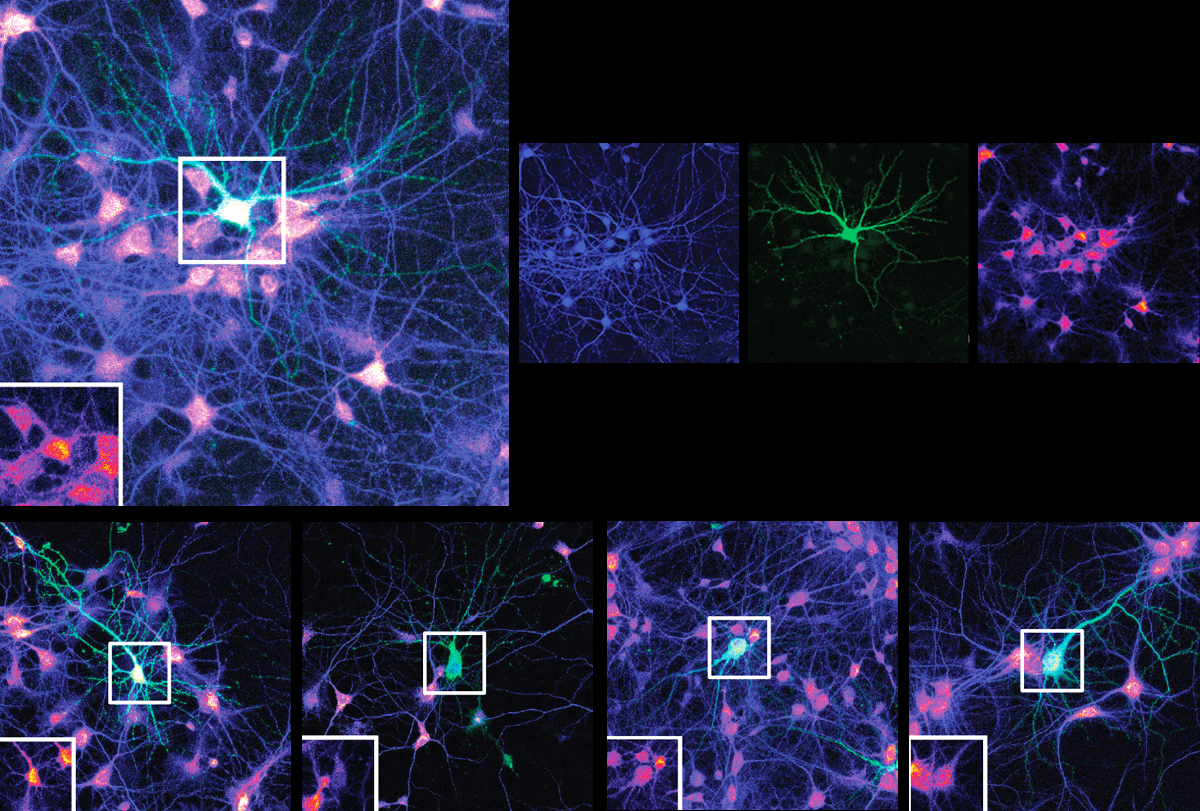
hepherd and his colleagues extracted brain tissue from young mice reared in a cage filled with toys, an environment known to boost plasticity and increase ARC expression. ARC proteins isolated from the tissue were directly bound to IRSp53, the team found.In a petri dish, purified ARC proteins formed more capsids when IRSp53 was also present, suggesting that the interaction between the two proteins prompts capsid assembly. And IRSp53 helped to launch the capsids from active neurons: Silencing its expression in cultured mouse neurons led to fewer ARC-containing vesicles exiting the cells, the study found.
Further experiments revealed that long-term potentiation (LTP), the process by which synapses strengthen in response to neuronal activity, induces IRSp53 to assemble and release ARC capsids. Applying a chemical that artificially triggers LTP enhanced expression of IRSp53 and ARC in dendrites. And the dendrites formed fleeting dendritic branches containing both proteins, live-cell imaging showed. Soon after, ARC levels abruptly dropped, suggesting that the capsids had ejected into the extracellular fluid, Shepherd says.