The SYNGAP protein abounds at excitatory synapses and orchestrates the synaptic plasticity crucial for learning and cognition. But it does so without tapping its eponymous “GAP” enzymatic activity, according to a study in mice, published today in Science.
Instead, SYNGAP shapes plasticity by physically freeing up space for more neurotransmitter receptors, the study suggests.
“This is a really big advance in our understanding of how this protein works,” says Paul Jenkins, assistant professor of pharmacology and psychiatry at the University of Michigan, who was not involved in the study.
The work also has important implications for the development of treatments for people who carry mutations — linked to intellectual disability and autism — in the gene SYNGAP1, which codes for the SYNGAP protein, Jenkins says. “The enzyme targets are probably not going to be enough on their own.”
W
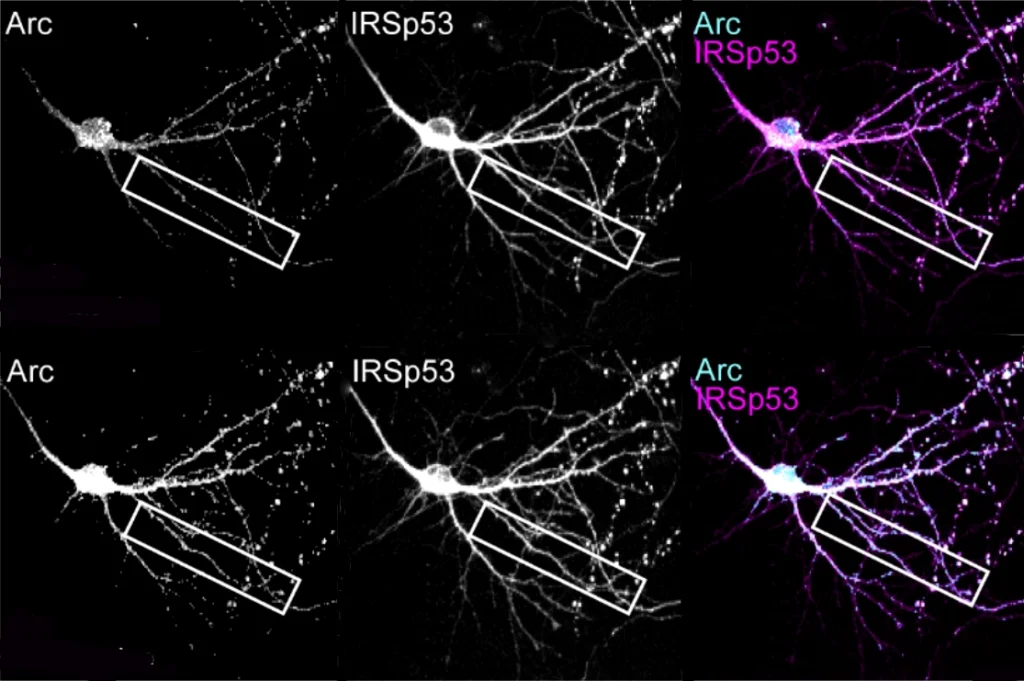
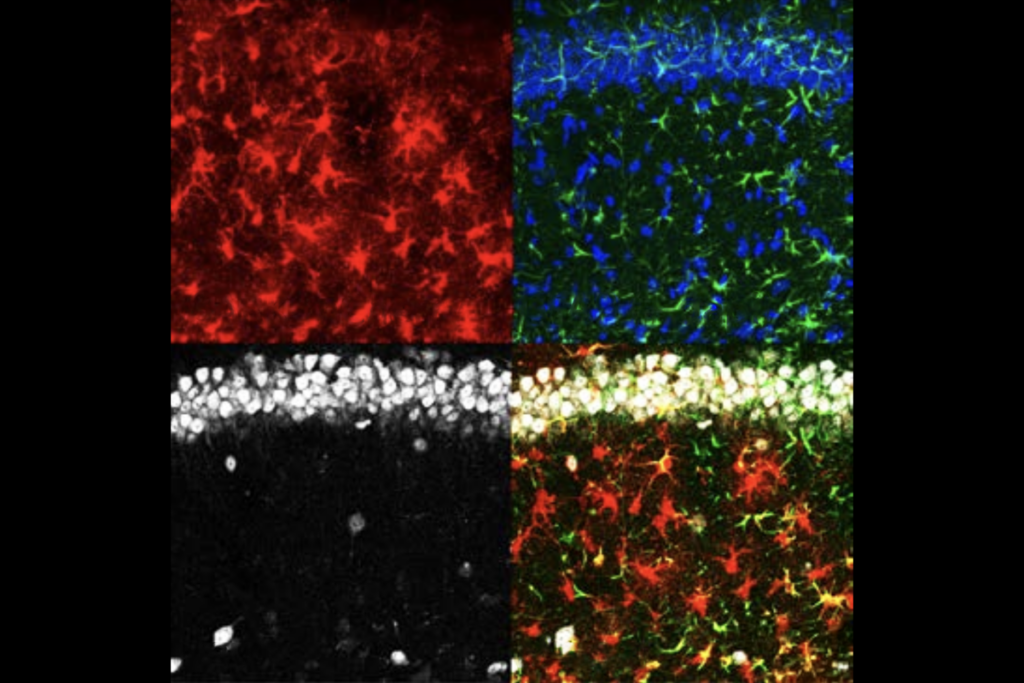
hen neuronal signals repeatedly activate a synapse, more AMPA receptors plug into its membrane to strengthen it, classic neuroscience studies have shown.SYNGAP is key for this process. It typically resides in a synaptic scaffold called the postsynaptic density, which anchors AMPA receptors in the membrane. There, its “GAP” domain serves to prevent more receptors from being added. When a neuron is activated, however, SYNGAP rushes out of the density, and that enzyme activity at the synapse dampens. In fleeing the postsynaptic density, SYNGAP also frees up spaces for the new AMPA receptors.
It was previously unclear which of these actions — SYNGAP’s reduced activity or its relocation — is responsible for the long-lasting synaptic changes.
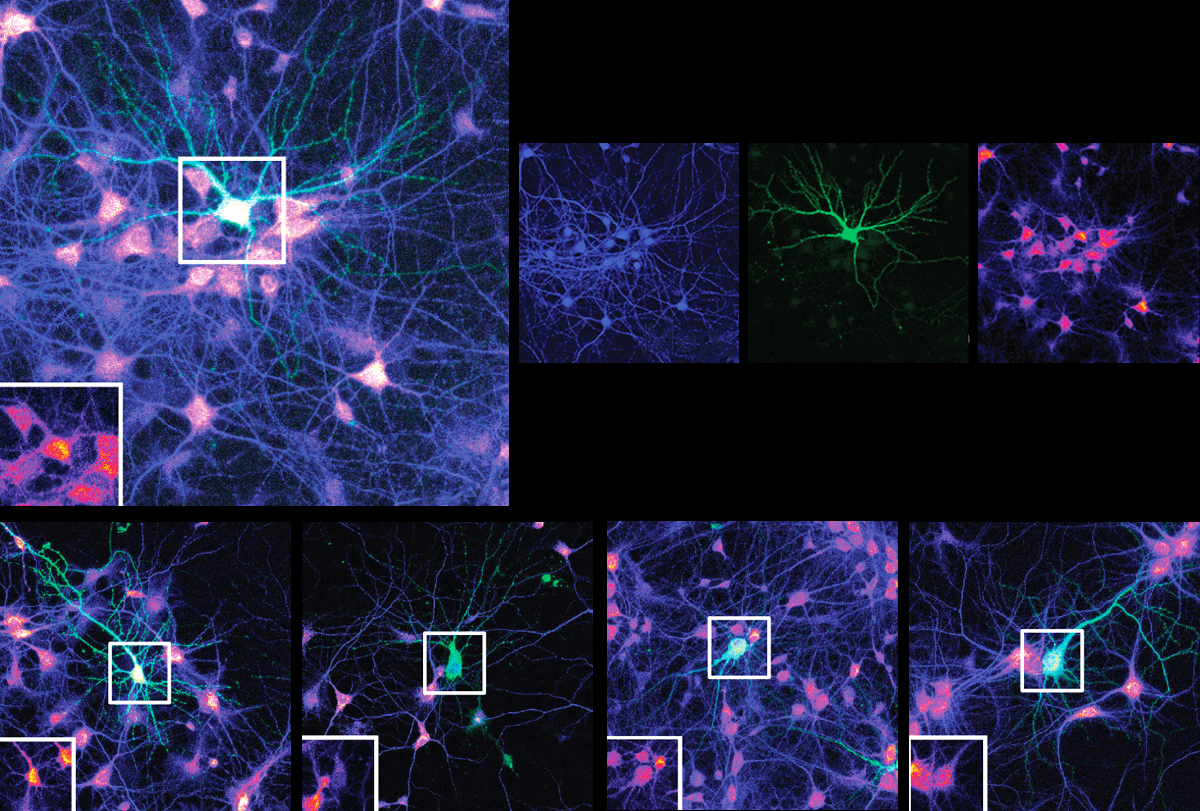
Experiments involving mouse neurons cultured in a dish point to the latter option, the new study shows: Synaptic strengthening worked as usual when the cultured cells expressed a form of SYNGAP with mutations in the GAP domain.
“That was our first proof of principle,” says lead researcher Richard Huganir, professor of neuroscience at Johns Hopkins University — and it convinced the team to engineer mice with mutations in the GAP domain in either one or both copies of the SYNGAP1 gene.
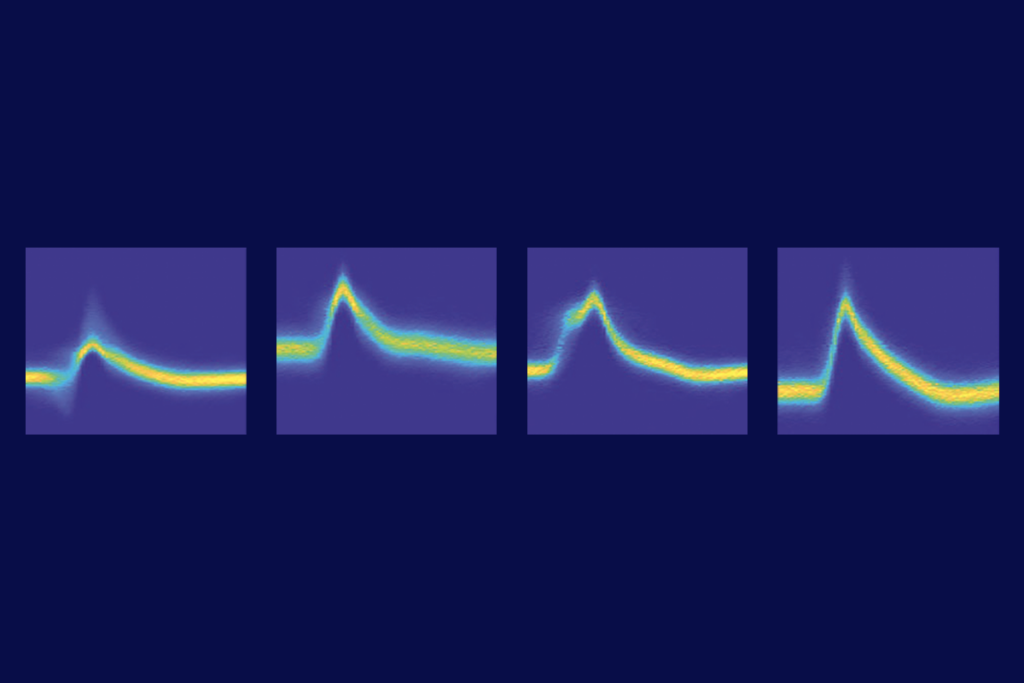
SYNGAP1-deficient mice survive for only a few days, are hyperactive and perform poorly on tests of working memory and fear-learning, but GAP-mutant mice appear to be no different from wildtype mice, the researchers found — suggesting that SYNGAP’s enzymatic activity is not crucial for those traits. The mutant mice also have typical synaptic plasticity and strengthening, recordings from the animals’ hippocampus showed.
“It’s surprising, and it’s an important result,” says Gavin Rumbaugh, professor of neuroscience at the UF Scripps Institute, who trained with Huganir but was not involved in the new study. “Everyone would have assumed — myself included — that you would see phenotypes in a GAP-dead mouse.”
Huganir and his colleagues uncovered further support for the idea that SYNGAP’s structural role governs synaptic plasticity — something he says that he and other researchers had suspected since noticing the protein’s unusual physical properties in 2016. The protein forms condensates — aggregates that behave like drops of oil in water — with the protein TARP gamma-8 and the scaffolding protein PSD-95. As those condensates form, SYNGAP and TARP gamma-8 compete to bind with PSD-95. And because AMPA receptors have to bind to TARP gamma-8 to be inserted into the membrane, that competition ends up regulating synaptic plasticity.
“SYNGAP here is acting like a gatekeeper,” Jenkins says, because there are only so many available slots in the membrane. “It’s blocking some of those spaces off. And when it leaves, it opens those spaces up for other proteins to come in — in this case, the AMPA receptor.”
Mutations that affect SYNGAP’s ability to form condensates hinder those gatekeeping abilities, Huganir and his colleagues found.
T
he work has important implications for how to treat people who carry mutations in SYNGAP1, Huganir says. People with these mutations have too little SYNGAP protein, which can result in epilepsy, intellectual disability and autism. Some therapeutics aim to correct atypical activity of the protein’s enzymatic targets, such as RAS. But the new results suggest that fixing that signaling is unlikely to help with synaptic plasticity or cognition.“It puts the nail in the coffin of treating RAS abnormalities in SYNGAP disorder,” Rumbaugh says. Instead, boosting levels of SYNGAP protein is likely a better goal.
The results also raise questions about the role of SYNGAP’s GAP domain, says Giorgia Quadrato, assistant professor of stem cell biology and regenerative medicine at the University of South California, who was not involved in the work. Its enzymatic activity may not contribute to the behaviors tested in the study but could still have other effects: The loss of that activity seems to alter early neuronal development, according to a study that she and Rumbaugh published last year.
Based on that work, and the fact that GAP activity is conserved across species, it seems unlikely that SYNGAP’s enzymatic function is unimportant, Huganir says. For instance, the GAP mutant mice may show other atypical behaviors that the team has not yet examined — something he and his colleagues plan to investigate next, he says. “[The mice are] normal in many aspects. But we still need to figure out what is normal and what is abnormal.”