The provocative concept of chronogeneity holds that autism involves a variety of developmental pathways, such that individual differences in traits and severity at any given point are only a snapshot of larger processes unfolding over time. If that theory is true, the heterogeneity that scientists typically seek to minimize through study design or analysis is actually key to understanding the condition.
A new study involving “a lot of different mouse genetic gymnastics,” as lead investigator David Ginty, professor of neurobiology at Harvard Medical School puts it, embraces this heterogeneity to pick apart one set of biological mechanisms by which diverse developmental pathways might arise.
Alterations in four different autism-linked genes interfere with the dampening of nerve impulses at varying points in development, Ginty and his colleagues found. The genes act on distinct parts of the neural circuit that carries touch information from the skin to the brain, with divergent consequences for the animals’ behavior.
“Overall, the paper is a tour de force,” says Carlos Portera-Cailliau, professor of neurology and neurobiology at the University of California, Los Angeles, who was not involved in the work. “They combine multiple approaches and explore mechanisms with in vitro electrophysiology. It’s very comprehensive.”
The results also add heft to previous findings from Ginty’s lab suggesting that some of autism’s behavioral characteristics have roots not only in the brain but also in the peripheral nervous system, where sensory alterations early in life can affect the wiring of the social brain.
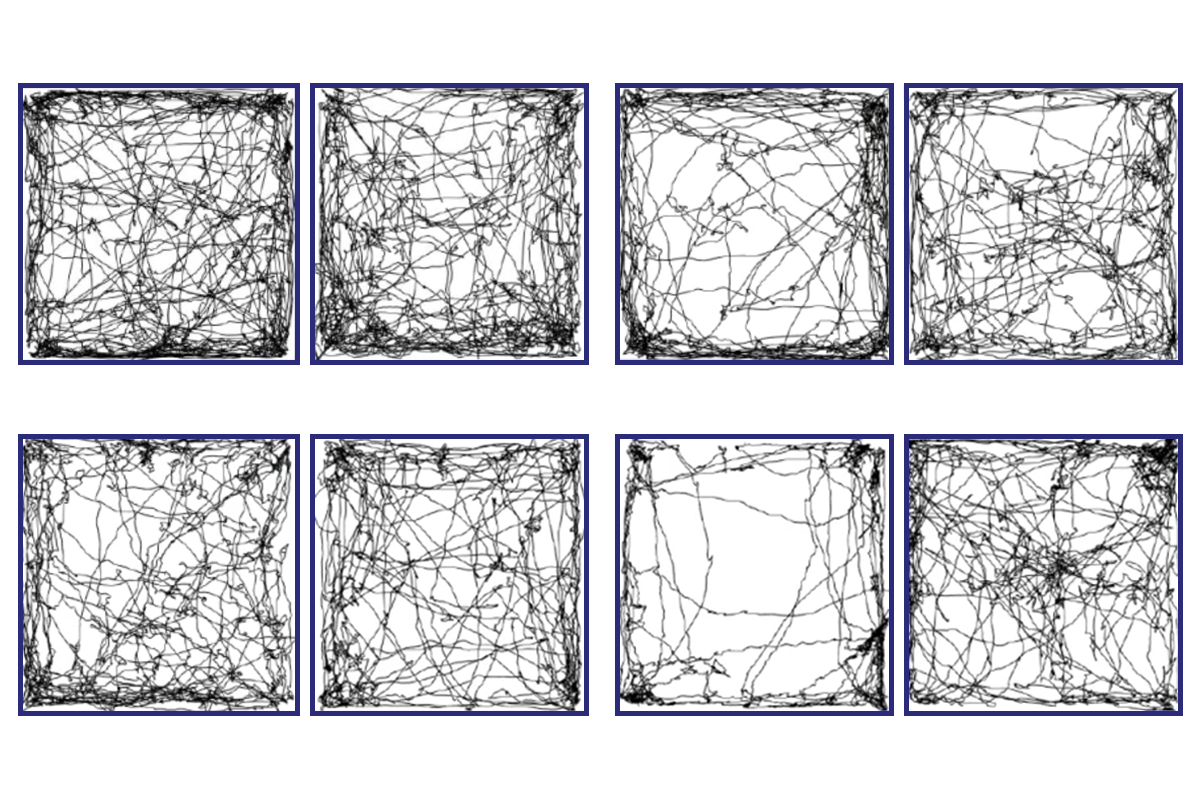
Mouse strains with disruptions in the genes GABRB3, MECP2, NLGN2 or RORB are hyperresponsive to touch as adults, the researchers report in the paper. But differences among them exist earlier in life.
A
t 4 days old, only the GABRB3 and MECP2 mice show touch sensitivity, the new work reveals. These mice move more than wildtype mice when a light puff of air hits their back. By contrast, NLGN2 and RORB mice respond no differently than their wildtype littermates at that age.“The phenotype is the same in adults, but you have different roots,” says Amaury François, a neuroscientist at the Institute of Functional Genomics at the French National Center for Scientific Research, who was not involved in the work.
A new optogenetic technique, which used light to activate touch receptors on the animals’ front paws, gauged touch sensitivity in even younger mice. GABRB3 mice are overreactive to touch on the day they are born, this method revealed.
What’s more, that pattern was already evident when the rodents were delivered via cesarean section at 18.5 days of gestation (a mouse pregnancy usually lasts 19 to 21 days). “It is very rare to see investigators perform behavioral experiments in animals just days old,” says Ishmail Abdus-Saboor, associate professor of biological sciences at Columbia University, who was not involved in the work.
The GABRB3 and MECP2 mice, which show touch oversensitivity early in life, also have social deficits and anxiety-like behaviors as adults. But the other two mouse strains, in which touch oversensitivity only develops later, do not. The results were published 17 January in Nature Neuroscience.
The findings align with previous studies from Ginty’s lab, Abdus-Saboor says. In that work, the researchers found that disrupting touch processing in the peripheral nervous system during a critical period early in development—but not later on—leads to autism-like behaviors in adult mice.
“With that said, I was surprised that timing mattered so much, in that what happens in the first days of life can manifest itself much later into adulthood,” he says of the new study.
T
he distinct developmental patterns linking tactile sensitivity to these genes reflect separate underlying mechanisms, Ginty and his colleagues also found. The neurons that pick up touch information in the skin, known as peripheral sensory neurons, are among the longest cells in the body.“They go all the way to the tip of your toe and project into the spinal cord,” Ginty says. In the spinal cord, the peripheral sensory neuron forms synapses with spinal neurons, some of which run messages up toward the brain.
At various points along this path, nerve cells have ways to dampen propagating signals. This inhibition helps the nervous system tune out extraneous information, such as the background hum of the refrigerator or the sensation of clothing against one’s body. When the nervous system can’t filter out the unimportant signals, sensory sensitivity—such as that often seen in autistic people—can result.
Additional mouse models in which autism genes were disrupted in only some nerve cells or parts of the body aided the researchers in teasing apart these mechanisms. GABRB3 and MECP2 contribute to dampening signals before they are passed from the sensory neuron to the spinal neurons, a phenomenon called presynaptic inhibition. Meanwhile, NLGN2 and RORB contribute to dampening signals that have already reached a spinal neuron, known as feedforward inhibition.
“Different modes of inhibition come online at different times,” Ginty says. The new work showed that presynaptic inhibition in this sensory circuit develops earlier in life than does feedforward inhibition.
That timing aligns with the emergence of sensory overreactivity associated with these autism-linked genes. “Perhaps the most interesting thing is that altering these different modes of inhibition can have different long-term behavioral consequences,” Ginty says.
The findings suggest different treatments for sensory sensitivity may be necessary, depending on its underlying mechanism, François says. About 60 percent of people with autism have altered sensitivity to touch, but, in line with the chronogeneity hypothesis, this characteristic may arise through different pathways—and require different strategies to be soothed.