Rett syndrome mutation’s effects vary by cell type
Mutations in the Rett syndrome gene MECP2 have different effects on subtypes of neurons.
Mutations in the Rett syndrome gene MECP2 have different effects on gene expression in subtypes of neurons. This variability could affect how the neurons respond to treatments.
Researchers presented the unpublished findings today at the 2017 Society for Neuroscience annual meeting in Washington, D.C.
Rett syndrome is a neurodevelopmental condition closely tied to autism. It stems from mutations in MECP2, a gene on the X chromosome. The mutation is generally lethal in boys, who have only one copy of the gene. But girls with Rett syndrome have one normal copy. Each of their cells expresses only one version of the gene.
MECP2 regulates the expression of other genes by attaching to DNA marked with tags called methyl groups. The pattern of methyl tags on DNA varies dramatically between cell types, so it is likely that MECP2 mutations have cell-specific effects.
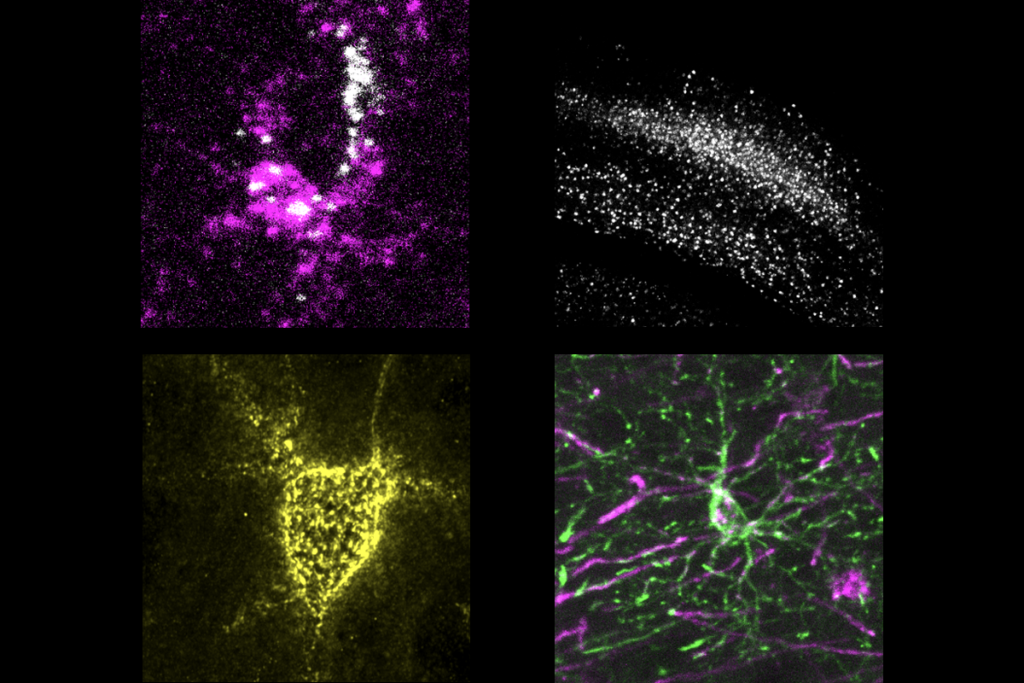
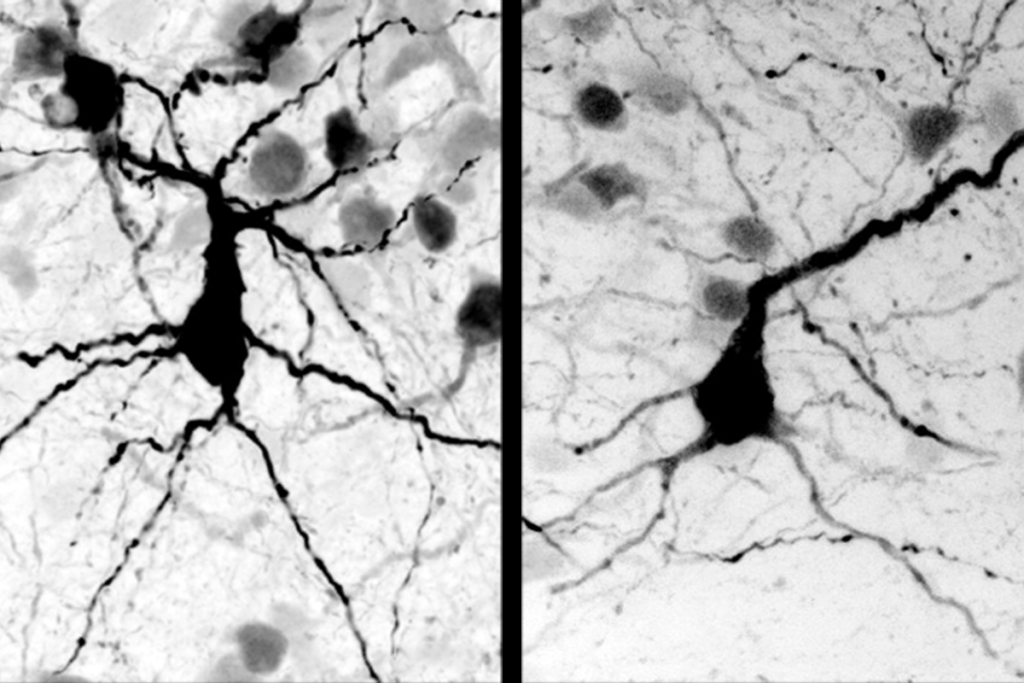
To test this, researchers studied postmortem brain tissue from three individuals with Rett syndrome. They examined the effects of MECP2 mutations on gene expression in neurons that boost brain activity, called excitatory neurons, and ones that dampen it, called inhibitory neurons. Patterns of gene expression in the two types of mutant neurons vary dramatically, the researchers found.
The findings have therapeutic implications for Rett syndrome. Targeting the molecular consequences of MECP2 mutations in neurons would mean treating “thousands of different effects across the brain,” says William Renthal, a postdoctoral fellow in Michael Greenberg’s lab at Harvard University, who presented the work. “Every cell has a unique disease,” Renthal says.
Technical trick:
To test their hypothesis, the researchers first had to isolate single cells from postmortem brain tissue and identify the different types. They plucked nuclei from the cells and used a technique called inDrop to sequence the RNA inside each nucleus. The technique attaches a barcode to each RNA molecule, indicating the type of cell the molecule came from. This enabled the researchers to distinguish between RNA sequences from excitatory neurons and those from inhibitory neurons.
They then had to determine whether each cell expressed the normal or mutant copy of MECP2 so they could focus on the relevant cells.
Single-cell RNA sequencing captures only certain regions of genes, so the researchers could not use inDrop to identify the mutant cells. To sidestep this problem, they identified genetic variants in the copies of MECP2 inherited from each of the donor’s parents that inDrop captured. This enabled them to figure out which copy of the X chromosome each cell expressed.
“We had to kind of create a ‘secret code,’” Renthal says. “That ended up solving the puzzle.”
The approach identified 1,714 genes that are either ramped up or tamped down in postmortem brain cells that express mutant MECP2. Genes with altered expression in one neuron subtype may have normal expression in the other. The list of affected genes includes ones implicated in autism, such as AUTS2, DAPK1 and RBFOX1.
Many are also altered in mice missing one copy of MECP2, a common animal model of Rett syndrome. “It looks like the molecular signatures we’ve been discussing in mice over the past several years really do translate into humans,” Renthal says.
The researchers plan to look at gene expression in other brain cell types, such as glia.
For more reports from the 2017 Society for Neuroscience annual meeting, please click here.
Recommended reading
Explore more from The Transmitter

Neuro’s ark: How goats can model neurodegeneration