The ability of amphibians to metamorphosize and, in some cases, regenerate limbs and even brain tissue raises puzzling yet fundamental questions about how a nervous system wires itself up. For example, if a frog’s legs don’t exist when its brain begins to develop—those limbs later replace its tadpole tail—how are the neural connections maintained such that, once the legs take shape, a frog can move them?
“How many connections are there between the spinal cord and the brain? How do they change over metamorphosis?” asks Lora Sweeney, assistant professor at the Institute of Science and Technology Austria.
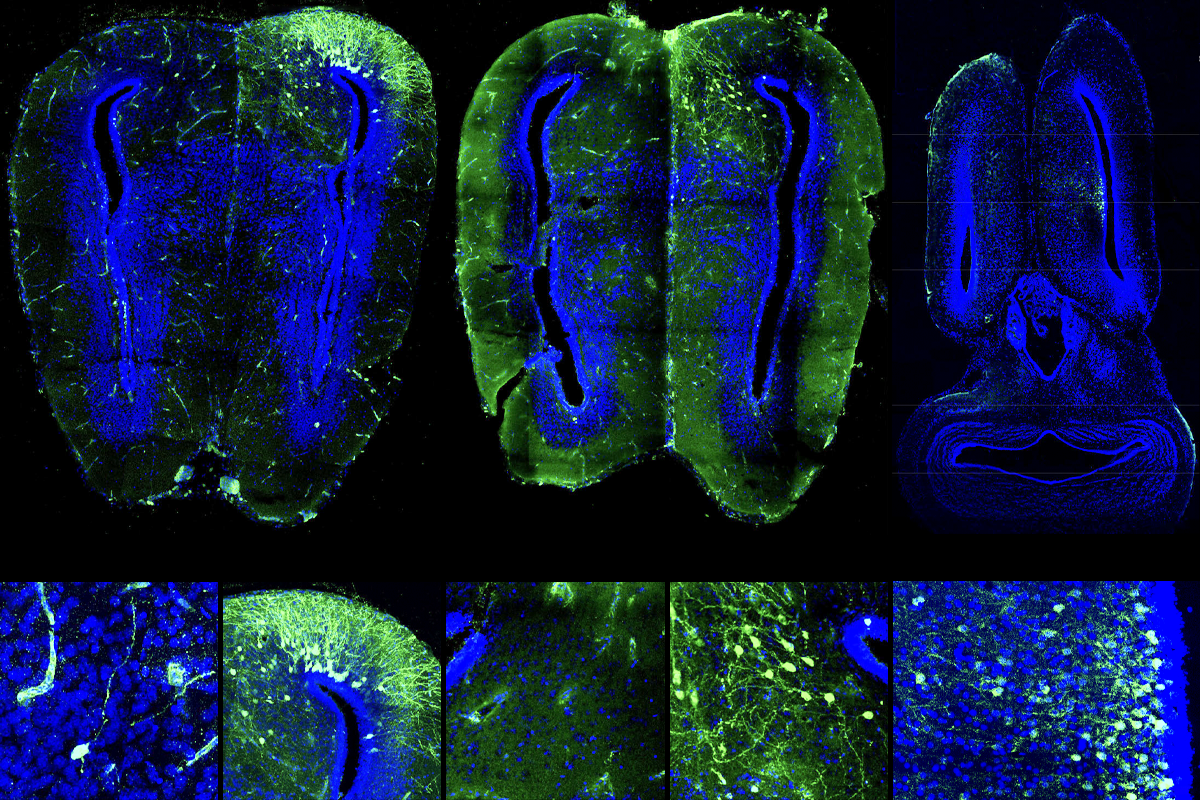
To find out, Sweeney and her colleagues decided to screen a panel of adeno-associated viruses (AAVs) in two species of frog and a newt. These viruses are commonly used to genetically manipulate brain cells in rodents and monkeys, but they have not been proven useful in amphibian experiments. With the right techniques, most common AAVs can deliver genes to amphibian cells through a process called transduction, according to Sweeney’s unpublished results, though the most effective viruses vary by species.
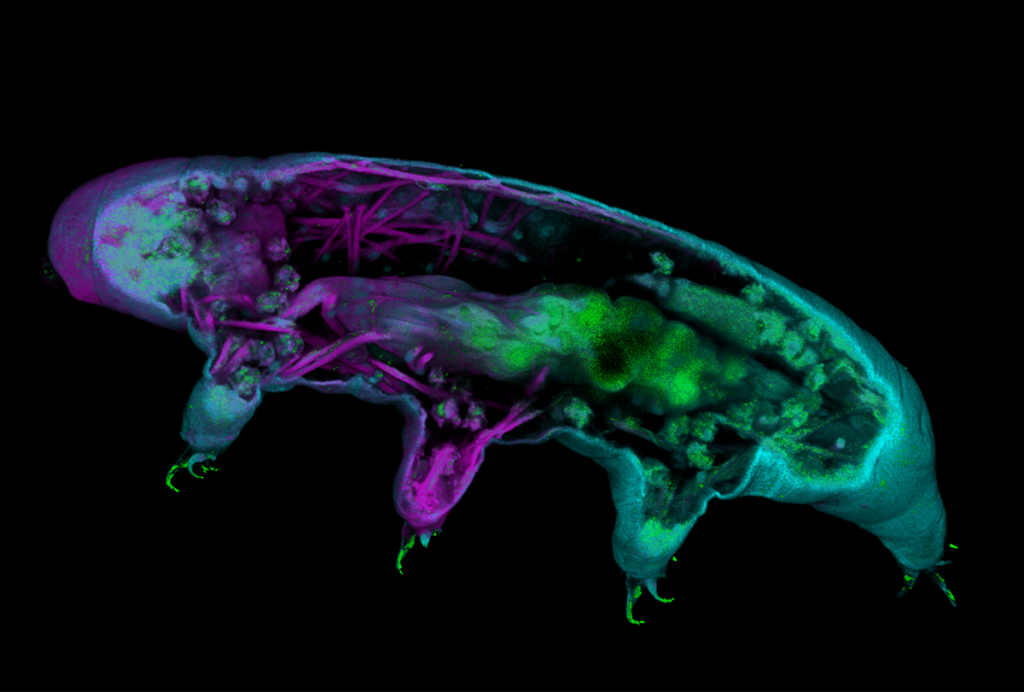
These amphibian-friendly AAVs can be used to trace neuronal connections and track groups of neurons born at the same time, the new work shows. And a subset of these same AAVs can also transduce cells in axolotls, newts’ fuzzy-gilled Mexican cousins, according to another preprint from an independent team. Both preprints were posted on bioRxiv in February.
“It’s a big game-changer,” says Helen Willsey, assistant professor of psychiatry at the University of California, San Francisco, who was not involved in either study but works with amphibian models. “It opens up a lot of doors for new experiments.”
O
ther researchers had previously tried to get AAVs to transduce cells in frogs and fish, with little success.“There was this idea in the field that perhaps aquatic species are not very good at taking up these viruses, because these are viruses that have been initially developed for mammalian research, from viruses that have mammals as hosts,” says Maria Antonietta Tosches, assistant professor of biological sciences at Columbia University, who co-led the study with Sweeney.
But she and her colleagues thought that it still might be possible with the right methodology. So they systematically tested six different AAVs at two different injection sites in African clawed frogs and Iberian ribbed newts, and three in Levant water frogs.
Although no single AAV worked best to transduce cells across all three species, the team was able to identify the optimal viruses for each species.
In the second study, the team screened seven AAVs for use with axolotls. All but one of the viruses could transduce the animals’ brain cells, and four were able to do so with high efficiency, the researchers found. “It was really a positive surprise,” says study investigator Katharina Lust, a postdoctoral researcher in Elly Tanaka’s lab at the Research Institute of Molecular Pathology. One AAV, AAV9, also worked to trace projections between the animals’ retina and brain, Lust and her colleagues found.
Three of the AAVs that work best in axolotls overlap with those that were effective for the Iberian ribbed newt—which is surprising, given the 150 million years of evolutionary distance between the two animals, Tosches says.
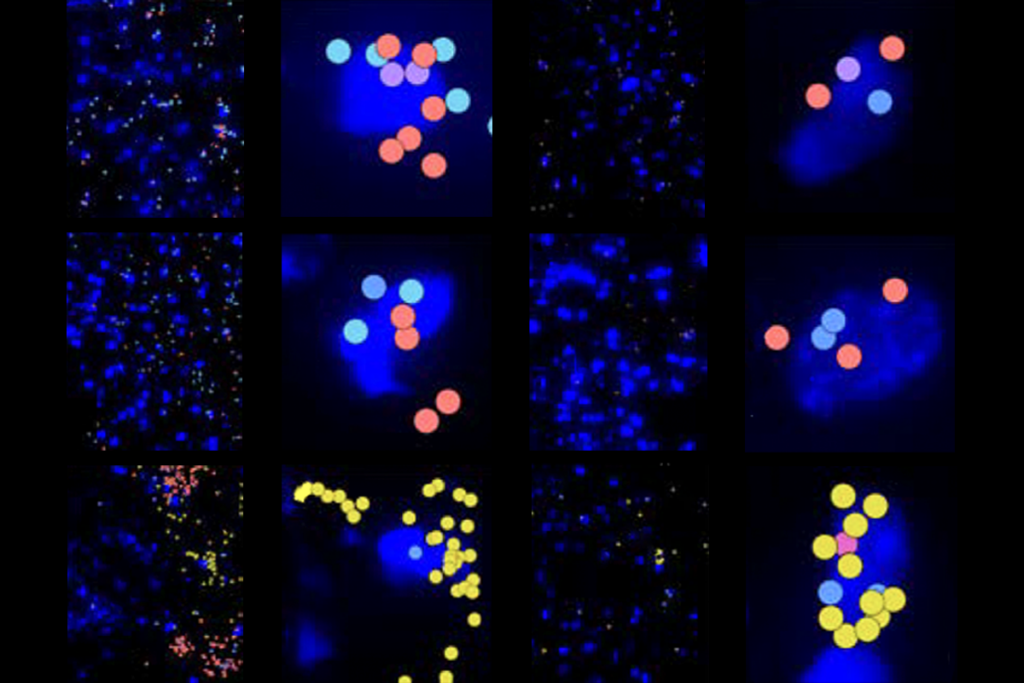
Some AAVs can transduce newt and frog cells both at the larval stage and after metamorphosis, Tosches, Sweeney and their colleagues discovered. The viruses can also drive the expression of a green fluorescent protein that renders the cells visible under a microscope. And AAVrg, which is known to spread retrogradely from one neuron to another, can label neuronal circuitry in frogs and newts. What’s more, virus injected into the animals’ ventricles during early development tagged specific subgroups of neurons that were being born at the time.
“Oftentimes in the nervous system, cells that are born at the same time share features within the circuit,” Sweeney says. “From a functional standpoint, it may provide us with a tool for manipulating neurons that share other properties.”
The new capabilities are important additions to the amphibian researcher’s toolbox, says Prayag Murawala, assistant professor at MDI Biological Laboratory, who was not involved in either new study but researches limb and tissue regeneration in axolotls.
Amphibian researchers previously had to use electroporation—essentially, electrocuting cells to take up new genetic material—which is highly inefficient for internal tissues, Murawala says.
Another option in the past was to engineer transgenic species, Murawala adds. But because most amphibians take years to reach sexual maturity—axolotls, for example, do not reach that stage until a year after birth—building up a colony takes a significant amount of time.
By contrast, AAVs can be produced in a couple of weeks in the lab, Murawala says. “And then all you have to do is inject—the virus does the job.”
T
o help others get started with AAVs, Tosches, Sweeney and their team are planning a workshop for nontraditional animal researchers who want to gain experience with this approach, and the preprint provides a detailed protocol for how to use the viruses, says Darcy Kelley, professor of biological sciences at Columbia University, who was a study investigator on the frog and newt preprint. “It ended up being a sort of how-to guide.”The new tools are exciting for the field, but it is not yet clear whether the AAVs can deliver gene-editing tools in these species, says Jessica Whited, assistant professor of stem cell and regenerative biology at Harvard University. And although the viruses have natural specificities for some cell types, the AAVs are not yet fully cell-type specific, she says. “It’s better than not having the tool, but it’s still not complete control.”
The team plans to test these additional capabilities in the future, Sweeney says.
Her next batch of experiments includes labeling neuronal populations in tadpoles and studying how much those connections change once the animals undergo metamorphosis, she says. And Lust and her colleagues aim to use the tools to track how neurons get rewired when an axolotl regenerates its brain tissue, Lust says.
“We hope to do circuit tracing, optogenetics and all those manipulations that people routinely do in mice,” Tosches says. “We want to do some modern neuroscience using amphibians.”