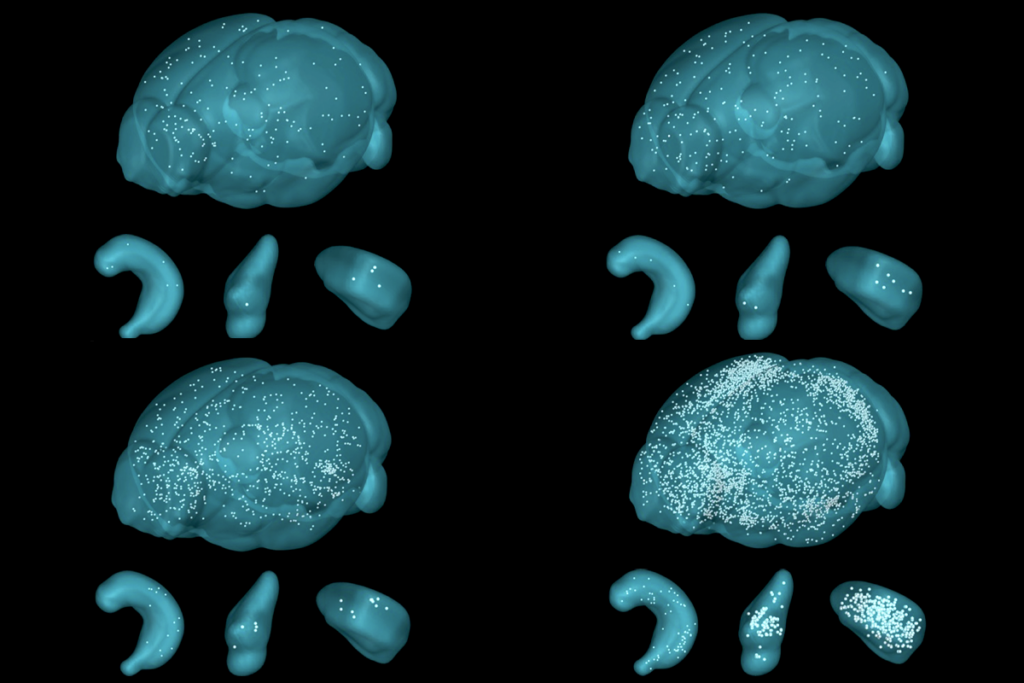
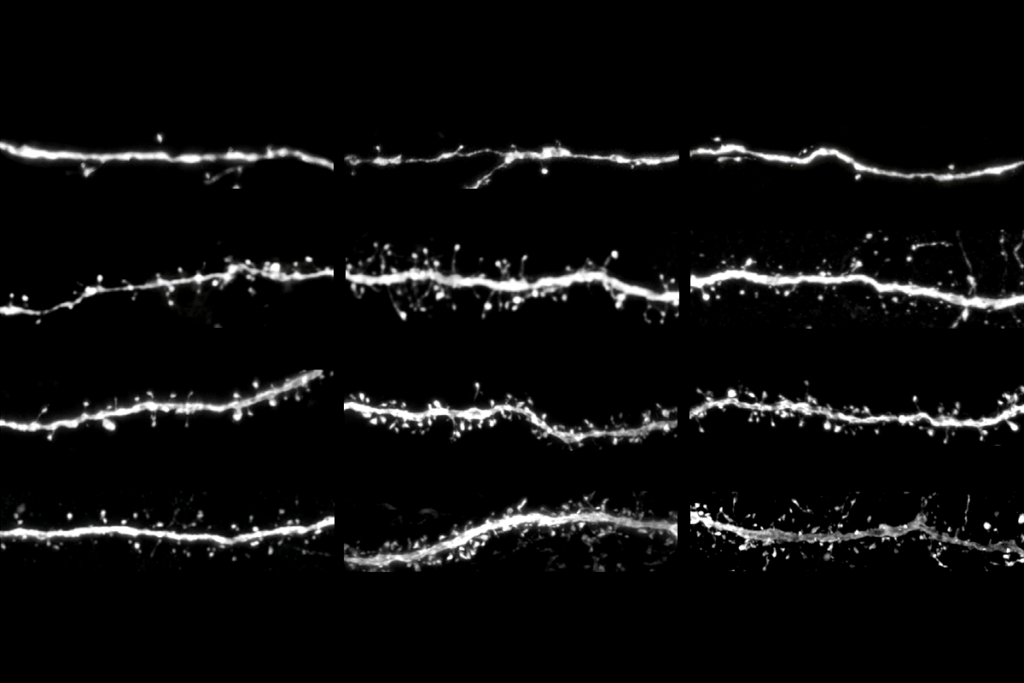
Memories are avid travelers: After they form in the hippocampus, they pass through intermediate regions such as the thalamus before finally stabilizing in the cortex. Yet not all memories survive this journey and become consolidated. The ones that persist do so thanks to a cascade of transcriptional changes in a thalamocortical circuit, a mouse study published today in Nature shows.
The work suggests that long-term memory is enabled by the sequential activation of molecular cascades in regions beyond the hippocampus, says study investigator Priya Rajasethupathy, associate professor of neurosciences and behavior at Rockefeller University.
The study also provides insight into what happens long after a memory is formed, says Hidehiko Inagaki, research group leader at the Max Planck Florida Institute for Neuroscience, who was not involved with the work. “People have studied a lot how expression changes overnight when the memory is first consolidated,” he says. “But here in this paper, they look at over many weeks—that’s a time scale that not so many people have studied mechanistically.”
Rajasethupathy and her team focused on the thalamus because previous work showed that connections stemming from this area mediate the maintenance but not the initial learning of a memory. “[The thalamus] is saying that something’s happening at the time of learning that’s assigning value, saying, ‘Hey, I want to be able to remember this in the future,’” she says.
As a mouse learns something new, neurons in the thalamus transition through multiple transcriptional states—each defined by a different expression profile and orchestrated by transcriptional factors that function as molecular timers for memory persistence, the work shows.
These waves of transcription show that memory formation and stabilization is not on or off but rather a sequential and dynamic process, Inagaki says. “Memory maintenance is not just a one-time thing, but it is a very active process which needs to keep happening,” he says. “It’s an active and continuous process.”
R
ajasethupathy and her colleagues created a virtual-reality task that enabled them to examine the differences between memories that are consolidated versus memories that are forgotten. During the task, thirsty mice navigated through a corridor until they reached areas in which they received a drink of water. The mice encountered one reward area frequently and another reward area less frequently.After five days of habituation and one week of training, the mice showed increased licking whenever they entered either reward area, indicating that they had learned to anticipate a drink of water in those locations, the study shows.