Phosphorylation of the metabolic enzyme pyruvate dehydrogenase inversely correlates with neural activity, according to a new study. This marker of decreased neural activity — the first ever described — doubles the data available to researchers who have long used c-FOS and other “immediate early genes” to pinpoint increased neural activity.
The phosphorylated pyruvate dehydrogenase (pPDH) marker fills a gap in basic neuroscience research, says lead investigator Li Ye, professor of neuroscience at the Scripps Research Institute. “The existing markers — the [immediate early genes] and the c-FOS — only tell you when the neurons are activated; and if the neurons stop being activated, or they are inhibited, that doesn’t report.”
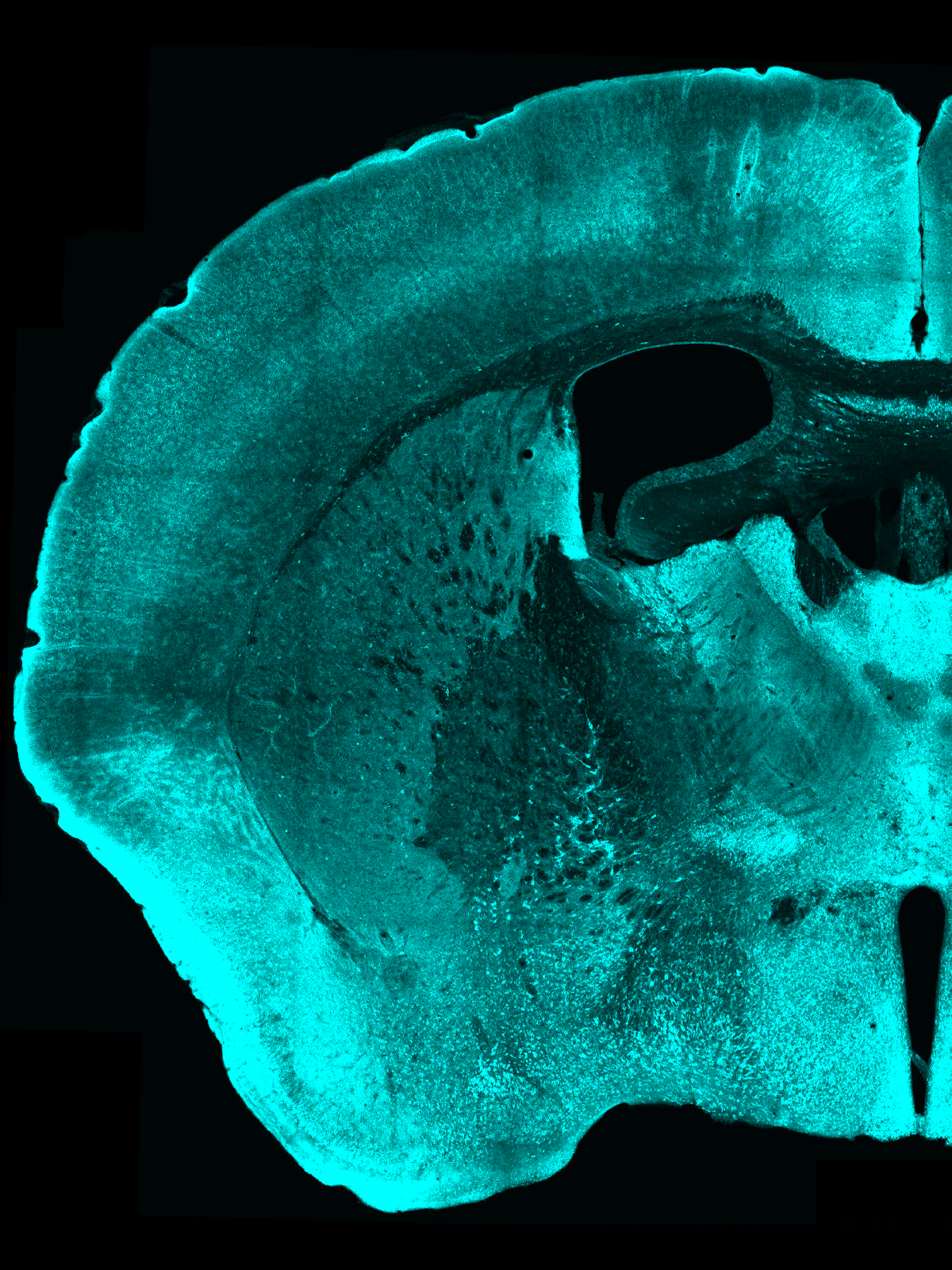
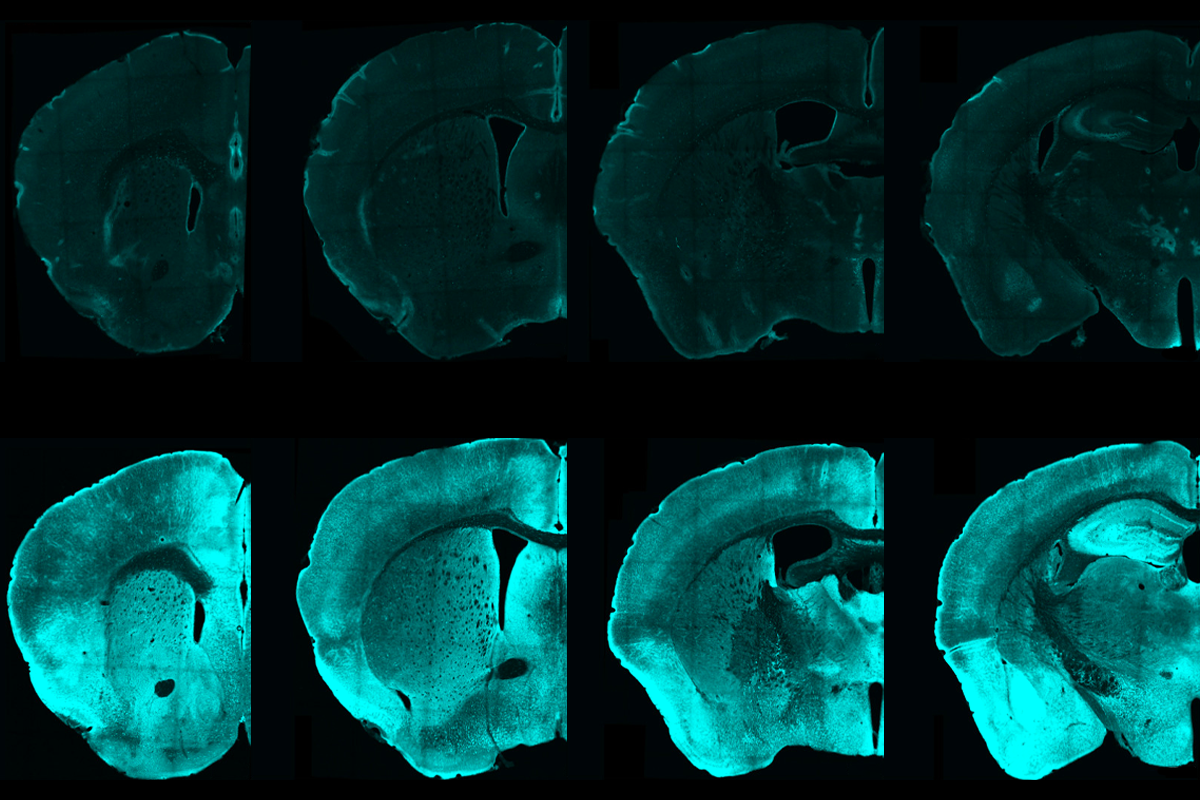
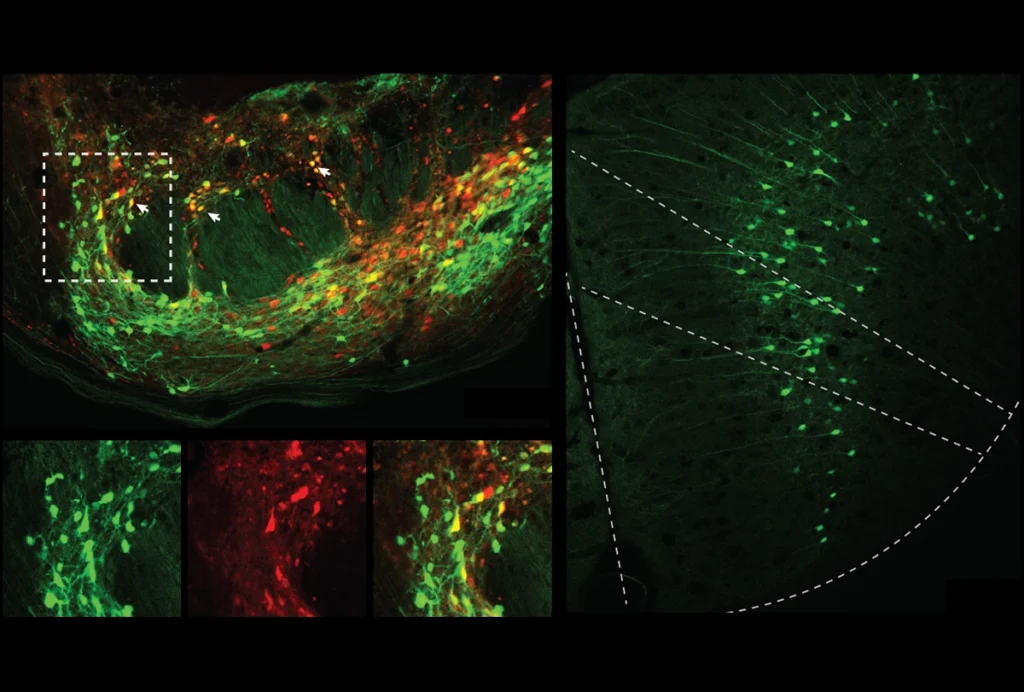
Now, washing fixed tissue slices with antibodies that target pPDH can light up those inhibited cells. By way of example, the team tested the method in mice temporarily lacking access to water and identified 10 brain regions previously unknown to be regulated by thirst.
Scanning for decreased neural activity can illuminate regions previously unidentified by c-FOS or other activity markers, says Vineet Augustine, assistant professor of neurobiology at University of California, San Diego, who advised the team on the thirst experiment. “This is a more holistic approach to looking at behavior readouts.”
T
he discovery, which took more than five years of work and combined optogenetics with proteomics screening, fulfills only half of Ye’s original goal: to identify a single marker that can track both the increase and decrease of neural activity.One key tool behind the breakthrough, Ye says, was an ultrabright LED light with enough power to stimulate action potentials in millions of primary cortical neurons transduced with channelrhodopsin. He and his team used the LED to make plates of cells fire in synchrony at either 0.5 hertz or 10 hertz. Afterward, they lysed the neurons and hunted for proteins that differed between the slow- and fast-firing groups.
Only one enzyme emerged: pPDH, which is involved in mitochondrial metabolism.
PDH is one of three subunits in the pyruvate dehydrogenase complex that kickstarts the Krebs cycle to produce energy for the cell in the form of ATP. When the PDH subunit is phosphorylated, though, ATP production stops. Low neural firing — which requires less ATP — might correlate with high levels of pPDH, the authors hypothesized.
Coincidently, a commercial antibody matching pPDH became available soon after the team flagged it as a potential marker, Ye says. Antibody in hand, the team verified that pPDH levels increased after they suppressed spontaneous neural activity in cultured neurons and in mice following anesthesia. The marker lit up nearly all regions of the anesthetized mouse brain.
It was at this point that study investigator Dong Yang, also at the Scripps Research Institute, says he realized that the tool would “attract the interests of many scientists who focused on different brain regions.”
Proving that pPDH correlates with behavior was more difficult, Ye says. “The cells don’t get inhibited when you do the opposite things to activation.”