Julie Forman-Kay is program head in molecular medicine at the Hospital for Sick Children in Toronto, Canada. She received her B.Sc. in chemistry from the Massachusetts Institute of Technology and her Ph.D. in molecular biophysics andd biochemistry from Yale University. The major focus of her lab is to provide biological insights into how dynamic properties of proteins are related to function and methodological tools to enable better understanding of dynamic and disordered states. Most recently, her lab has probed the biophysics of protein phase separation and how it regulates cellular condensates and biological function.

Julie Forman-Kay
Program head
Hospital for Sick Children
From this contributor
How microscopic ‘condensates’ in cells might contribute to autism
A controversial idea about how cells compartmentalize their contents into droplets — like beads of oil in water — could be key to understanding autism, says Julie Forman-Kay.
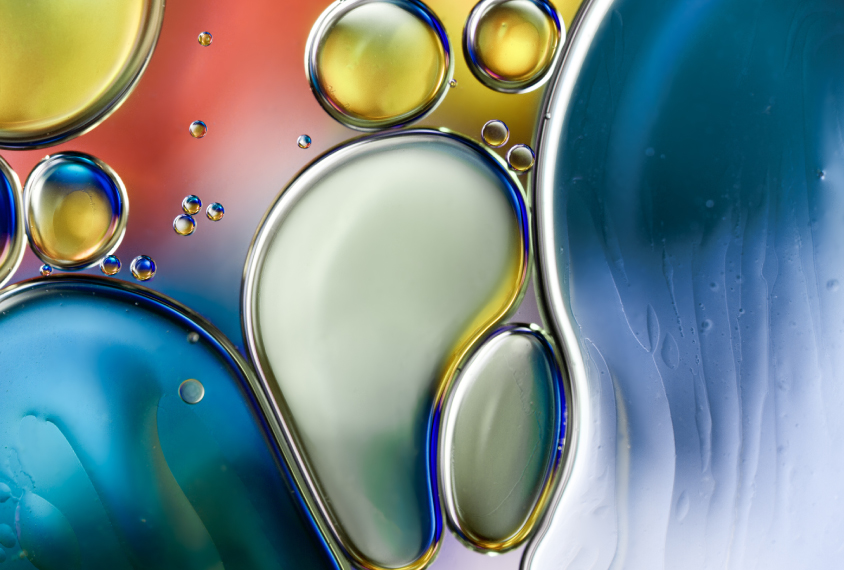
How microscopic ‘condensates’ in cells might contribute to autism
Explore more from The Transmitter
Securing the academic pipeline amid uncertain U.S. funding climate
Finding creative ways to keep early-career researchers in academia—for example, through part-time roles—can help the field weather the storm.

Securing the academic pipeline amid uncertain U.S. funding climate
Finding creative ways to keep early-career researchers in academia—for example, through part-time roles—can help the field weather the storm.
Let’s teach neuroscientists how to be thoughtful and fair reviewers
Blanco-Suárez revamped the traditional journal club by developing a course in which students peer review preprints alongside the published papers that evolved from them.
Let’s teach neuroscientists how to be thoughtful and fair reviewers
Blanco-Suárez revamped the traditional journal club by developing a course in which students peer review preprints alongside the published papers that evolved from them.
New autism committee positions itself as science-backed alternative to government group
The Independent Autism Coordinating Committee plans to meet at the same time as the U.S. federal Interagency Autism Coordinating Committee later this month—and offer its own research agenda.
New autism committee positions itself as science-backed alternative to government group
The Independent Autism Coordinating Committee plans to meet at the same time as the U.S. federal Interagency Autism Coordinating Committee later this month—and offer its own research agenda.