Functional MRI (fMRI), though expensive, has many properties of an ideal clinical tool. It’s safe and noninvasive. It is widely available in some countries, and increasingly so on a global scale. Its “blood oxygen level dependent,” or BOLD, signal is altered in people with almost any neurological condition and is rich enough to contain information specific to each person, offering the potential for a personalized approach to medical care across a wide spectrum of neurological conditions.
But despite enormous interest and investment in fMRI — and its wide use in basic neuroscience research — it still lacks broad clinical utility; it is mainly employed for surgical planning. For fMRI to inform a wider range of clinical decision-making, we need better ways of deciphering what underlying changes in the brain drive changes to the BOLD signal.
If someone with Alzheimer’s disease has an increase in functional connectivity (a measure of synchrony between brain regions), for example, does this indicate that synapses are being lost? Or does it suggest that the brain is forming compensatory pathways to help the person avoid further cognitive decline? Or something else entirely? Depending on the answer, one can imagine different courses of treatment. Put simply, we cannot extract sufficient information from fMRI and patient outcomes alone to determine which scenarios are playing out and therefore what we should do when we observe changes in our fMRI readouts.
To better understand what fMRI actually shows, we need to use complementary methodologies, such as the emerging optical imaging tool of wide-field fluorescence calcium imaging. Combining modalities presents significant technical challenges but offers the potential for deeper insights: observing the BOLD signal alongside other signals that report more directly on what is occurring in brain tissue. Using these more direct measurements instead of fMRI in clinical practice is not an option — they are unethical to use in people or invasive, requiring physical or optical access to the brain. But recent advances in rodent and non-human primate fMRI make it possible to use multiple complementary technologies simultaneously in animal models. Researchers can make tightly controlled observations across an animal’s lifespan and test novel intervention strategies, which will be especially important for fMRI to become an effective clinical tool.
W
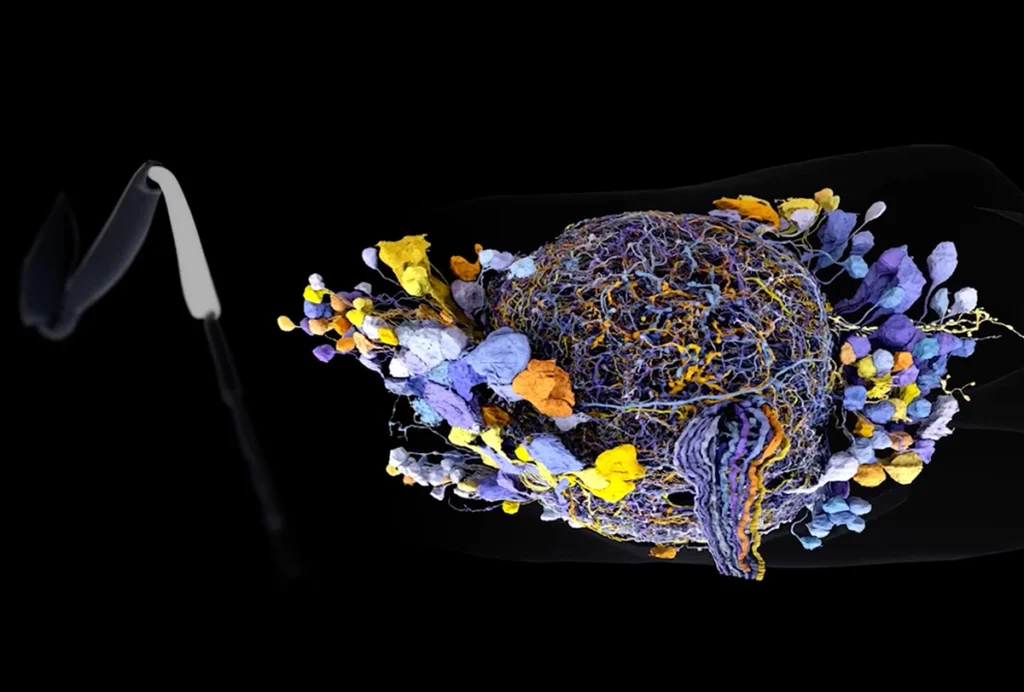
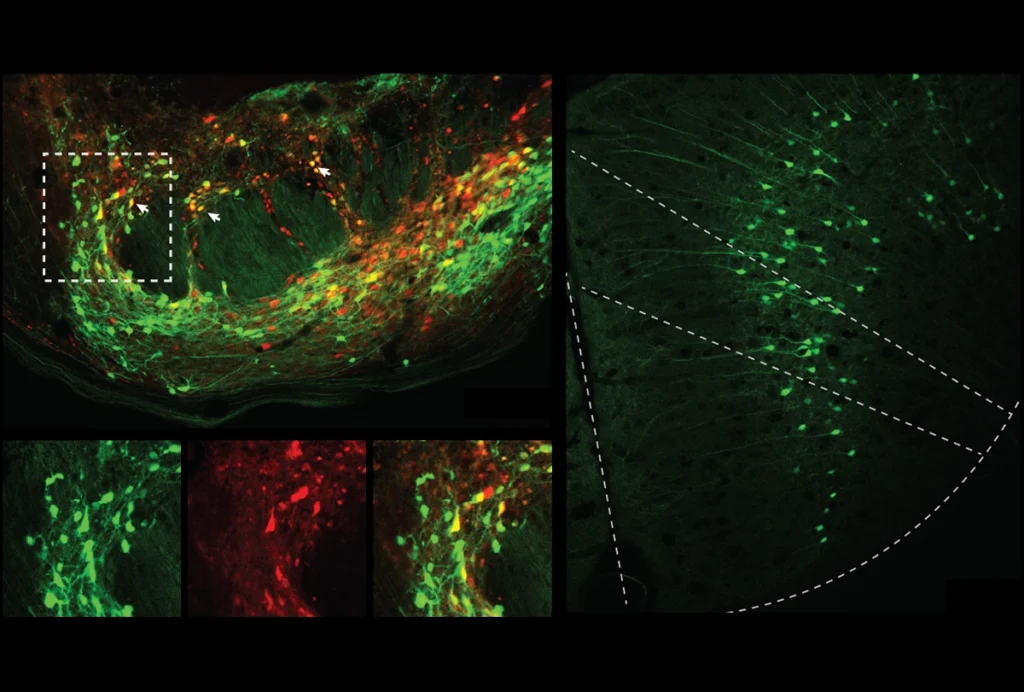
ide-field fluorescence calcium imaging is particularly promising as a complementary technology in that it encompasses a large field of view — in mice, the entire cortical mantle — enabling the researcher to monitor brain activity at the circuit or network-level. It overlaps well with the whole-brain view afforded by fMRI and can be targeted to specific cell types. In combining these two modalities, we can simultaneously observe cell-type-specific activity and the clinically accessible (but indiscriminate) BOLD fMRI signal.My group has built a device capable of simultaneous wide-field optical imaging and fMRI, which we are using to track the emergence and progression of dynamic changes in functional connectivity in a mouse model of Alzheimer’s disease. In research published in Nature Communications in January, we showed that the two methods track well overall, with BOLD signals in line with excitatory neural activity. We also observed some intriguing instances where the two modalities diverged, which may reflect an uncoupling between neural activity and vascular responses.
Our work and that of others hints at the promise of multimodal imaging. But to realize that potential, the field needs to overcome a number of challenges. Obtaining high-quality fMRI data from animals requires an array of skills that go well beyond that required to collect human fMRI data — among them, basic-to-complex animal care (including breeding and genotyping); surgical manipulations and post-op recovery; anesthesia; intubation/extubation and ventilation; animal training (for imaging awake subjects); maintaining animal physiology during imaging; data manipulation and curation; statistics; neurobiology; engineering; disease and injury modeling in animals; machine learning; study coordination; specialized software and hardware development; clinical medicine and inter-species translation; and more. Proficiency in these skills requires years of training and practice, and the number of labs with expertise to give this training is small compared with those doing more traditional human fMRI. For high-quality multimodal data, the demands are even greater, including expertise in both imaging modalities, engineering and their combination.