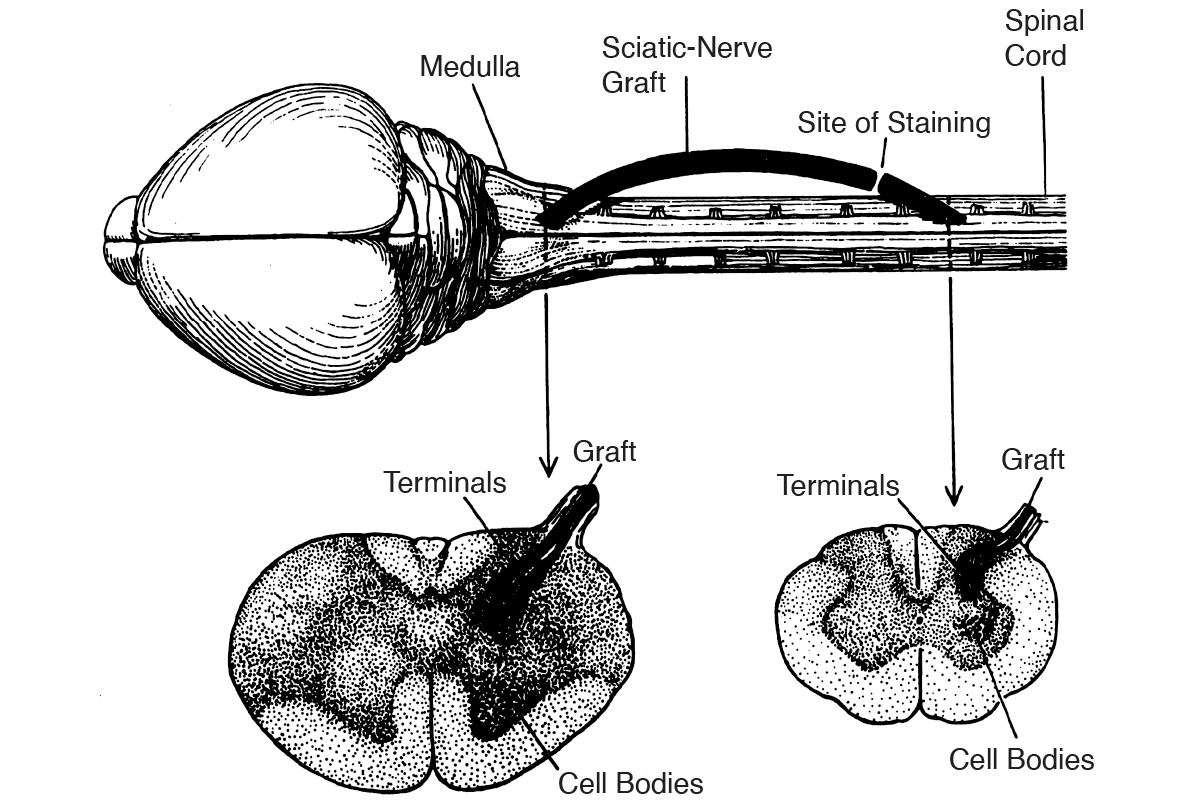
Further, Aguayo’s findings suggested that glia found in peripheral nerves (Schwann cells) were different from glia in the brain and spinal cord (oligodendrocytes). Other experiments showed that peripheral neurons could not grow in the presence of CNS glia. Based on these observations, it appears that the ability to regenerate after injury is dependent on the exact glia components in the environment of neurons.
Following David and Aguayo’s experiments, two other hypotheses about regeneration have gained attention. The first theory points to a lack of trophic factors, such as NGF, which would prohibit regeneration. Since trophic or growth factors have been known to stimulate regeneration in the periphery, perhaps these factors are not present in the CNS, and this is the reason for a lack of regeneration. Paul Patterson (1900–65), a professor of biology at Caltech, speculated that “the lack of regeneration in the CNS is due to the absence of the necessary stimulatory or permissive substrates.” From 1960 to 1990, growth factors present in the CNS were investigated and debated, even though purifying the proteins in the nervous system was an arduous task. Eventually better ways of detection became available and showed that the same growth factors in the periphery (such as NGF, EGF, and insulin) are also present in the brain and spinal cord. So the inability to regenerate is not due simply to a lack of growth factors.
The second theory posits that a lack of growth may be due to a physical or chemical block, such as a “glial scar” barrier known to develop after nerve injury, or from an accumulation of inhibitory substances. In an initial attempt to repair the tissue damage, the local environment reacts by producing a network of proteins called the extracellular matrix. The net result is to produce a new physical environment that blocks nerve cells from regenerating. After a spinal cord injury, there is myelin debris and a number of cell types that are damaged—immune cells, astrocytes, and oligodendrocytes—which constitute the glial scar. This kind of environment is not conducive to regeneration. Before many others, Paul Patterson realized that “there was a growing list of potential cases of glia exerting an important influence on axon growth.” In fact, “CNS glial membranes were involved in inhibiting axon extension,” so Patterson was prophetic in promoting the concept of “the importance of being inhibited.” Forces from glia prevented regeneration.
To bring theory into practice, the most pressing question was, What cell types prevent regeneration (and in turn, which ones promote it)? It turns out that oligodendrocytes, the glial cells in the brain (not Schwann cells in the periphery), are a major cause of the problem. Axons assiduously avoid contact with oligodendrocytes because of specific proteins expressed on the surface of these cells; inhibitory cell surface proteins have a negative influence on regeneration in the spinal cord and the brain. One of the first proteins discovered by the Swiss scientist Martin Schwab was called “nogo,” to convey that lack of growth or inhibition. The theory that substances block regeneration has been verified with many other nogo-like proteins. Today we know that there are many inhibitory proteins expressed by oligodendrocytes and myelin that block regeneration. Not many of these proteins are found in Schwann cells.
Now this is a puzzle. So far, the theories that have been posited have not been proven. Perhaps we should put the previously stated question another way: Why do myelinating cells in the periphery promote regeneration but myelinating cells in the brain do not?
One possible explanation is the need to shape the morphology of neurons through inhibiting growth and the targets that are innervated. Another way of patterning cells in the brain is by a process called “tiling,” in which there is a self-avoidance and restriction of cells in a particular zone. (We will reconsider this problem in Chapter 8, on plasticity.) Further, because myelin continues to be made into late adulthood, it has long been thought that changes and remodeling of myelin may occur from experience and neuronal activity. Measurements to visualize white matter myelin have shown there is a great deal of variability as a result of experience and exposure to different activities, which is the base definition of plasticity. Neuronal activity can, in turn, regulate changes in myelin. Therefore, myelin plasticity may go hand in hand with neuronal plasticity. This notion comes from detection of changes in myelin thickness with an increased ability to perform motor tasks, such as piano playing and figure skating. On the other hand, other paradigms, such as stress, social isolation, and sensory deprivation, have a negative effect on myelination.
Keeping in mind our earlier discussion of myelin, the continuous formation of new myelin, the variability in its thickness, and the changes in the lengths between the nodes of Ranvier—all intriguing properties that suggest myelin is much more than a covering, or glue, of axons. The detection of gradients and changes in myelin profiles has stimulated the search to find out if myelin remodeling could be responsible for the acquisition of new motor tasks, new learning, and increased cognition.