Sensory issues associated with autism may be caused by fluctuating neuronal noise — the background hum of electrical activity in the brain — according to a new mouse study.
Up to 90 percent of autistic people report sensory problems, including heightened sensitivity to sounds or an aversion to certain smells. Yet others barely register sensory cues and may seek out sensations by making loud noises or rocking back and forth.
But thinking in terms of hyper- or hyposensitivity may be an oversimplification, says Andreas Frick, lead investigator and research director at INSERM. “It’s becoming clear now that things are a lot more nuanced.”
For instance, the brain’s response to visual patterns — measured using electroencephalography (EEG) recordings — varies more between viewings in autistic people than in those without the condition, one study found. And functional MRI has detected similar variability among autistic people, suggesting sensory problems may arise from inconsistent brain responses.
In the new study, Frick and his colleagues found variability in the activity of individual neurons in a mouse model of fragile X syndrome, one of the leading causes of autism. That variability in neuronal response maps to fluctuations in the levels of noise in the brain, the study found.
Noise within the brain isn’t necessarily a bad thing. In fact, an optimum amount is ideal: a little can give neurons the ‘push’ they might need to fire an action potential, while too much can make it difficult for the brain to distinguish between different stimuli. But in animals modeling fragile X syndrome, noise fluctuates such that they process sensory information less reliably, Frick says.
“It’s an exciting and ambitious study,” says Elizabeth Milne, professor of cognitive neuroscience at the University of Sheffield, who led the EEG study but was not involved in the new work. It allows scientists to move beyond speculation that neuronal noise drives the inconsistent brain responses seen in autistic people, she says.
F
rick’s team worked with mice lacking FMR1, the gene responsible for fragile X syndrome. Using a technique called patch-clamp recording, they monitored the activity of individual neurons in the somatosensory cortex — the brain region that processes touch — as they repeatedly tapped the rodents’ paws.In wildtype mice, neurons tended to respond in a similar way each time their paw was touched. But neurons from rodents lacking FMR1 showed greater variability in the size of the electrical signal, and how long it took to initiate a response, the study found.
“It’s nice that they use hindpaw stimulation as it adds a translational component” says Anubhuti Goel, assistant professor of psychology at the University of California, Riverside, who was not involved in the study. Past research has often opted for whisker stimulation, which is clearly not relevant to people, she says.
The scientists then measured how much the electrical activity along the neuron’s membrane changes when the cell is unstimulated. This “baseline membrane potential” varies twice as much in fragile X mice as it does in wildtype rodents. And the more neuronal noise fluctuates, the greater the variation in the brain cell’s response to touch, the study found. That diminished signal-to-noise ratio might be responsible for the neuron’s “jittery” response to sensory signals, Frick says.
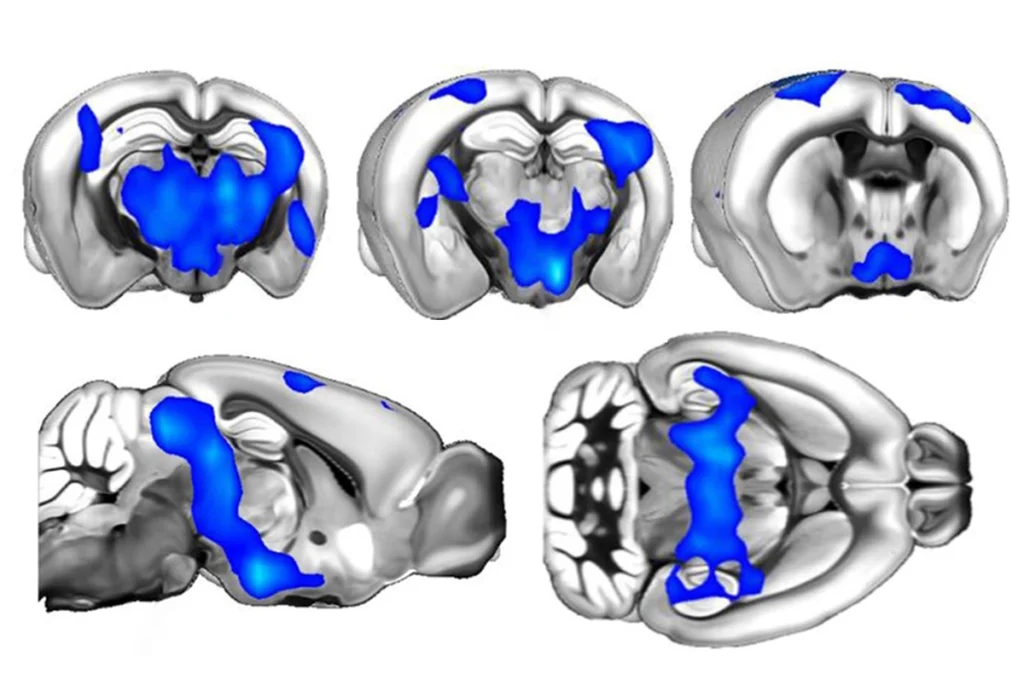
The neural oscillations — known as brain waves — that contribute to specific brain states also vary more in mice missing FMR1 and correlate with variability in neuronal response, the researchers found. These results hint that disorderly brain waves — which have been linked to autism — may contribute to sensory issues.
The team then treated the animals with a compound that activates a potassium ion channel in the neuronal membrane. The ion channel is regulated by FMRP — the protein product of FMR1 — and may contribute to the hyperexcitability of neurons in mice lacking the protein, previous work has shown.
A
ctivation of the ion channel stabilizes membrane voltage, causing neuronal activity in the mice missing FMR1 to somewhat resemble that seen in wildtype mice, the study found. Attempts to rescue sensory problems by “playing with the noise” in this way have not been done before, Frick says.The findings were published 30 November in Nature Communications.
Because the mice were anesthetized, the researchers couldn’t tell whether any of the neuronal differences seen in fragile X mice lead to changes in behavior. But data from previous work — which measured the rodents’ reaction to a mild sound — revealed that animals missing FMR1 displayed more variability in their behavioral response than wildtype mice. The findings suggest that inconsistent neuronal activity may lead to more diverse behavior in autism, the researchers say.
Some researchers speculate that variability in sensory processing may drive the social problems seen in people with autism. “Imagine being at a party, talking to a friend while music is playing in the background,” Frick says. “You have to integrate the sound of their voice with the movement of their lips.” But if there’s variability in sensory processing, socializing will be more challenging, he says.
Although the theory is appealing, the researchers The Transmitter contacted were cautious about drawing a link without the evidence to back it up. “My view is that this link isn’t particularly well defined or evidenced in current literature,” Milne says.
Still, the new work goes a long way to explain the variability seen in EEG recordings of autistic people, she adds.
But other concerns remain. Recordings from individual neurons don’t reflect the variable brain responses seen in people, says Cian O’Donnell, lecturer in data analytics at Ulster University, who was not involved in the work. “For the activity of a single neuron to be detectable at the scalp, it would need to be synchronized among many neurons. For me, that’s a missing link between the human and the animal data.”
Frick and his team plan to plug that gap by recording populations of neurons using two-photon imaging. They also aim to uncover whether variability in sensory processing affects social behavior in mice, which could support or debunk the connection to core autism traits.