Over millions of years, the retina evolved in vertebrates to suit the needs of different species. But even though its function held steady — taking in light to form interpretable images, much like a camera — how its underlying parts had changed, or not, over time was unclear. Only humans and other primates, for example, were thought to have midget retinal ganglion cells, suggesting these cells evolved for primates’ specific use.
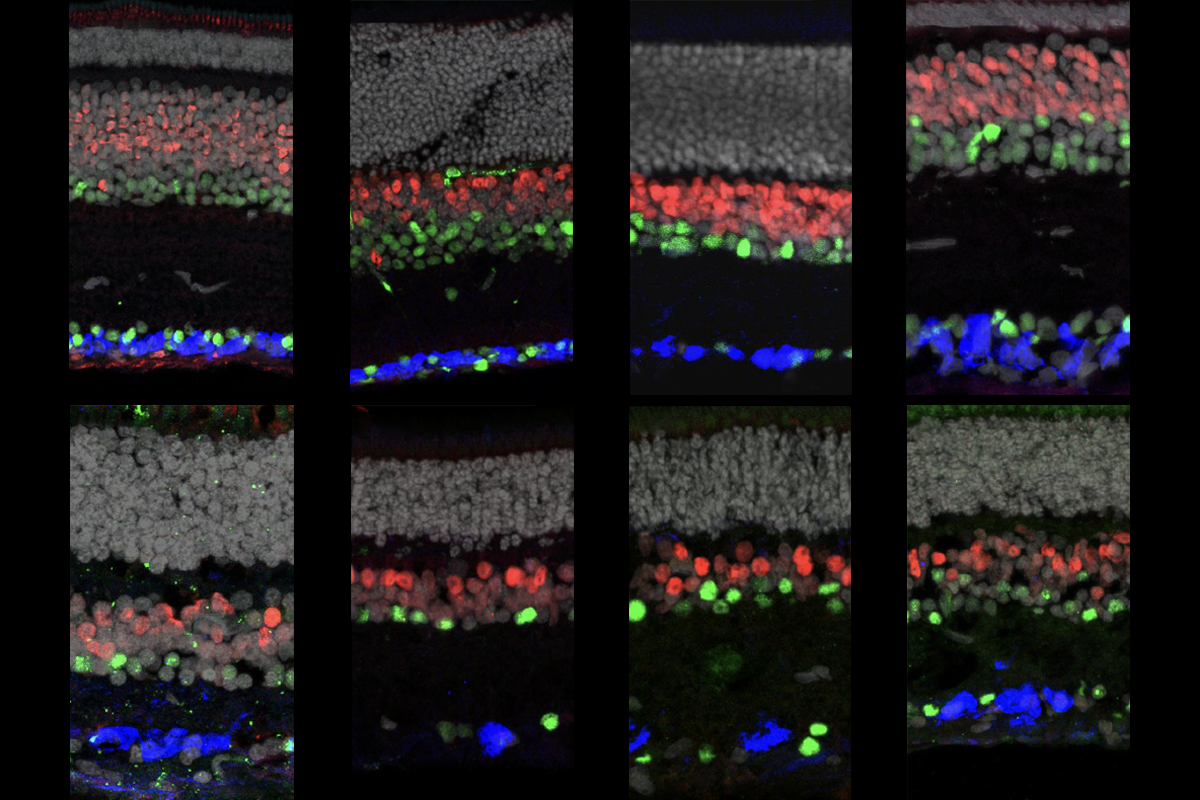
As it turns out, retinal cell classes are highly conserved, according to the RNA transcripts they express. The basic classes of cells present in the retina — including photoreceptors, bipolar cells and retinal ganglion cells — are conserved across 17 vertebrate species, from humans to cows to lizards, according to a new study published in Nature on Wednesday. Midget retinal ganglion cells, the work reveals, have mouse analogs. And primates have a type of ganglion cell previously thought to be found only in lower vertebrates, according to another study, which appeared in Nature at the end of October.
The findings suggest that each cell class performs the same role across species and that variations in cell types can address different functional needs, says Joshua Sanes, professor of molecular and cellular biology at Harvard University and co-lead investigator on the study published this week.
“Primates haven’t had to reinvent the wheel,” says Teresa Puthussery, assistant professor of optometry and vision science at the University of California, Berkeley, and lead researcher on the October study. “We’ve simply changed the allocation of our resources.”
M
idget retinal ganglion cells have small receptive fields, which enable high-acuity vision. These cells, which feed the cortex fine-grained information about the visual field, constitute more than 80 percent of the human retina’s ganglion cells — the cells that send signals directly to the rest of the brain. Mice, on the other hand, have a wide variety of ganglion cells — none of which look immediately comparable to human midget cells based on their morphology.The transcripts present in retinal cells across species, including mice and humans, tell a different story, however. “There was a very clear relationship — and we could trace it,” Sanes says.
A type of mouse ganglion cell, called the sustained alpha cell, is not unlike the human midget cell, the team found. Both versions form dendrites in similar locations, are innervated by the same kind of cell and project to the same part of the brain. But the cells serve different functions: In primates, these cells are small and numerous, and they offshore their processing to the cortex; in mice, they are large and sparse, and that processing happens locally, thanks to the wide variety of ganglion cells present.
“We’ve been using ancient parts, but those parts have been tinkered with,” says Karthik Shekhar, assistant professor of chemical and biomolecular engineering at the University of California, Berkeley and co-lead researcher on this week’s study. “Like an old engine that’s been modified.”
The cells’ shared roots provide important context for translational research — such as the study of glaucoma, which stems from problems with midget retinal ganglion cells. “It means that we can transfer insights from work on non-primate species much more readily to our own eyes than previously thought,” says Tom Baden, professor of neuroscience at the University of Sussex in the United Kingdom, who was not involved in the study.
The amount of species variation among cell types is highest among these retinal ganglion cells, according to the work by Sanes, Shekhar and their colleagues. That suggests that the evolutionary pressure is strongest for cells that send signals out of the retina to the rest of the brain, Sanes says.
“You can find the same elements in different species, independent of in which environment they live. But they took on different jobs,” says Thomas Euler, professor of ophthalmic research at the University of Tübingen in Germany, who was not involved in the study. “That illustrates how flexible the system is on an evolutionary level.”
B
ut how did those different jobs came about? One theory, Sanes says, is that the retina and the cortex evolve alongside each other.Mice, for example, may need to have quick reflexes in response to simple visual stimuli — a darting prey or a looming predator — so a large portion of their visual processing can happen in the retina. That explains why they have a higher variety of ganglion cell types, too, Sanes says.
Humans, on the other hand, are less reliant on rapid responses. “We can respond maybe 50 milliseconds slower than a mouse,” which gives the cortex time to process visual signals, Sanes says. “But because we can use our cortex and be flexible, we make up for speed in craftiness.” It’s similar to a trade-off between having a complex camera that can preprocess an image and having an advanced graphics card that can do those computations after the image is already captured, he says.
That idea is sometimes put as “the dumber the animal, the smarter the retina,” Euler says. But, he adds, not everyone is convinced. “The complexity of the retina also depends on the environment of the animal,” he says.
For instance, macaque monkeys have a subtype of retinal ganglion cell that responds to a specific direction of visual motion and sends signals to the brainstem to generate appropriate gaze-stabilizing eye movements, according to the October study by Puthussery and her colleagues. That subtype, called ON-type direction-selective ganglion cells, had not been previously seen in primates. But by analyzing clusters of transcriptomic data, Puthussery and her colleagues were able to guess their identity and then test that prediction with functional experiments. The subtype enables rapid processing of visual information in the monkey retina, they found — which suggests that this sort of reflex is still important for animals with a powerful visual cortex.
Sanes, Shekhar and their colleagues confirmed the presence of these cells by comparing their transcriptomic data across species. Additional analyses like that one could help provide a common reference for the field that guides further studies of retinal cell types, Puthussery says.
Those insights may also benefit neuroscience research more broadly, Baden says. Retina research is often ahead of the curve, thanks to its conserved structure and experimental accessibility, he says. “What we see in the retina today, we are almost sure to see in other parts of the brain tomorrow.”