Almost 20 years ago, a Chilean lab published a curious result about the degu, a guinea-pig-sized rodent native to the Andes. The team compared the brains of young degus with those of old ones and reported that the brain of every old degu contained amyloid-beta plaques and tau protein deposits—in other words, the hallmark signs of Alzheimer’s disease pathology.
The animal, the team concluded, could be used to model sporadic Alzheimer’s disease, in contrast to the transgenic mouse models researchers had already created to mimic the inherited, early-onset version of the disease. And an animal that lives long enough to develop the disease pathology naturally, such as the degu, they asserted, could prove more relevant for translational research.
Nibaldo Inestrosa, professor of biological sciences at the Pontifical Catholic University of Chile, who led the initial degu work, continued to build the case through a series of follow-up studies. But then, in 2016 and 2017, results from two European labs presented a strong rebuttal: They found no signs of Alzheimer’s disease pathology in the brains of older degus.
Patricia Cogram watched this debate unfold and began her own degu experiments in 2010. “We never believed the story that all the degus, when they get old, they will have Alzheimer’s,” says Cogram, associate professor of genetics at the University of Chile. “That was slightly a crazy idea.”
But she had a hunch about why the European results had differed so dramatically. After talking to several investigators with degu colonies around the world, she learned that their laboratory animals had been “super-hyper-inbred” for decades. She opted to use only wild-caught animals in her own work, she says. “We thought, no one has done genetics with this animal. So we will never cross or inbreed the animal until we understand what is going on.”
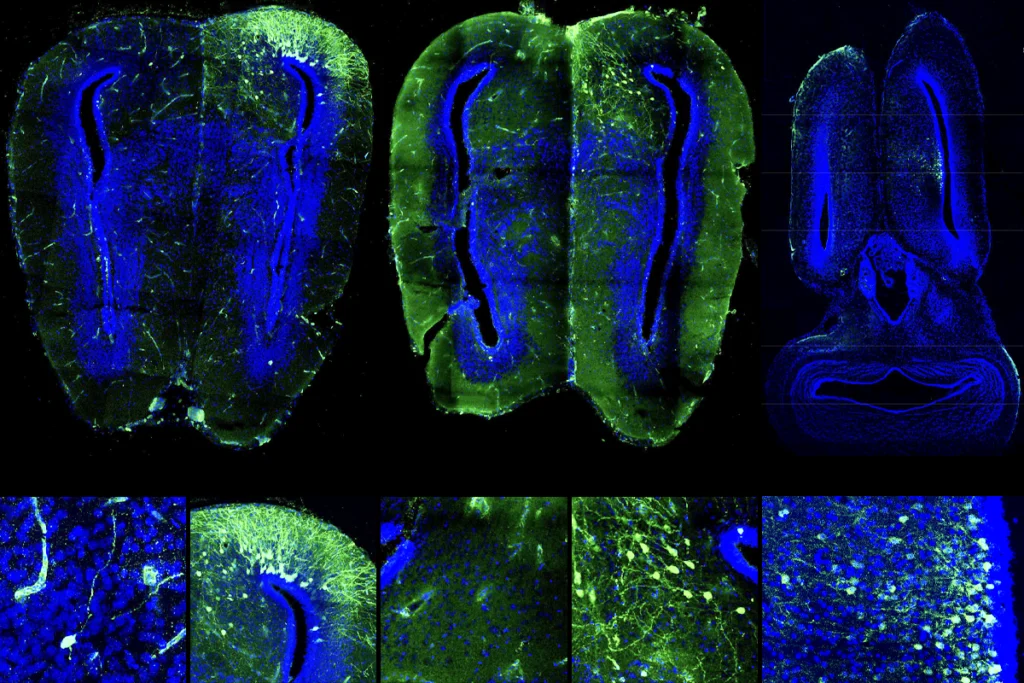
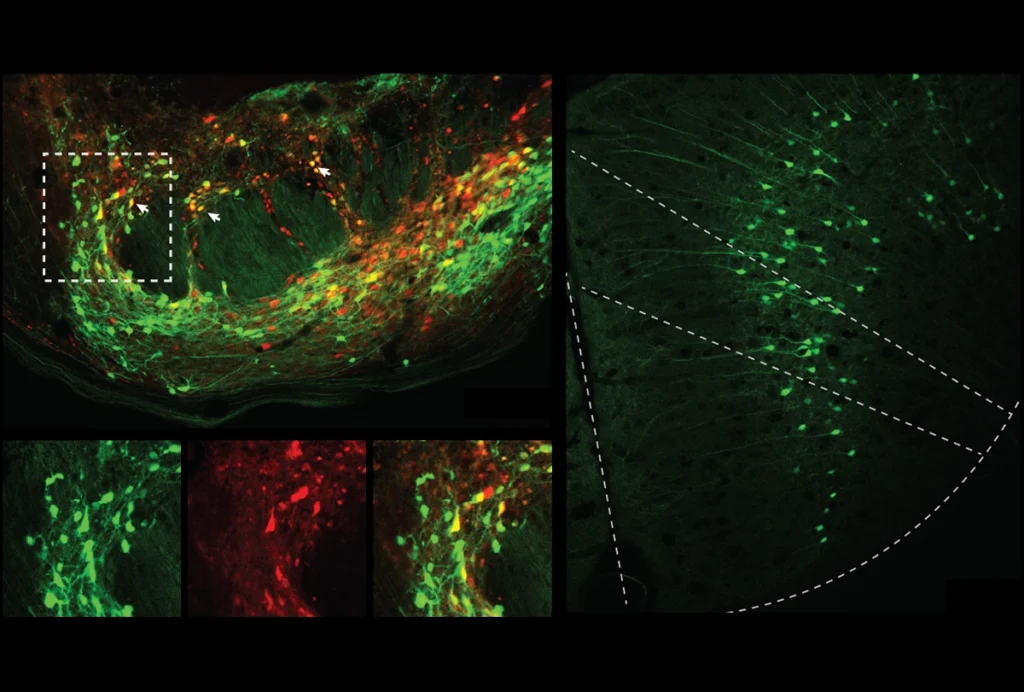
Her decision paid off: In 2022, Cogram and her collaborators found plaques and tau deposits in some, but not all, older degus. The degus with Alzheimer’s disease pathology—about a third of the animals—also performed worse than the others on cognitive and behavioral tests.
The resulting paper “cleared the field,” says Manuel Moro, health scientist administrator at the U.S. National Institute on Aging (NIA), who oversees projects on alternative animal models of Alzheimer’s disease. “I’m extremely positive and happy with that paper.”
Now the degu is garnering support as an animal model for Alzheimer’s disease. Cogram and a U.S. collaborator secured a $5 million, five-year grant from the NIA to develop a single-cell atlas of the animal and compare its pathology more directly with that of humans and other models. And this past December, Moro and about two dozen researchers gathered in Chile for a conference on the degu and other unconventional animal models of Alzheimer’s disease. “We have high expectations of the degu,” Moro says.
But if Cogram hadn’t tried to crack the inbreeding problem, the degu model might not have made it off the ground.
T
he first degus used for research in the United States descended from 20 animals brought into the country in 1964, according to archival news stories in The Burlington Free Press. A farmer caught the animals in a rural town about 20 miles outside of Santiago, Chile, on behalf of a scientist at the Massachusetts Institute of Technology. The colony grew to 87 degus before MIT donated it to David Boraker, a professor in the medical school at the University of Vermont, in 1970.Boraker studied the degu’s immune system and kidneys and also used it as a multipurpose disease model: In addition to amyloid plaques, degus also naturally develop cataracts, diabetes and high blood sugar. Under Boraker’s care, the colony grew to about 900 animals, including a portion funded by the National Eye Institute for cataracts research. By 1978, the Vermont colony had supplied more than 30 other research institutions across the U.S. with animals.
The colony no longer existed by 2000, says Theresa Lee, then professor of psychology and neuroscience at the University of Michigan. Lee studied circadian rhythms and was shopping around for a diurnal rodent to raise in the lab when she came across a 1975 zoology paper by Boraker that described the degu. She assembled a colony from scratch, obtaining degus from pet stores and a zoo in Illinois that was shutting down; an ecologist who did field work in Chile provided her with some wild degus.
When Lee tried to replicate Inestrosa’s original findings with her own colony, she found no plaques in the animals’ brains. She never published her results, but like Boraker, she sent animals to other researchers who wanted to start up their own colonies—and many of the degus used for research in the U.S. and Europe likely descended from Lee’s colony, Cogram says.
“There was a period of time when I think I might have been the only colony outside of a zoo in the United States,” Lee says. “And I was giving away degus like crazy. Everybody wanted degus to start their work.”
This distribution pattern could explain why the original degu studies produced opposite results, Cogram says: Decades of inbreeding skewed the gene pool of Inestrosa’s colony toward Alzheimer’s pathology, and the European colonies away from it.
W
hen Cogram started her own degu studies, she was “quite obsessed on getting things right,” she says. In addition to paying close attention to the genetic makeup of her colony, she partnered with Robert Deacon, a rodent behavior researcher at the University of Oxford, to develop a suite of behavioral tests tailored specifically to the degu.The studies that ignited controversy over the degu as a model of Alzheimer’s disease had all measured cognitive decline using mouse behavioral tests. In the object-recognition test, for example, young healthy mice display a preference for new objects over familiar ones and spend more time investigating objects they don’t recognize. In mice, this preference typically declines with age, but degus have better eyesight than mice because they are diurnal. And so because they can recognize objects from farther away, Cogram says, their preference to explore new objects may go unnoticed when compared with mice.
When Lee attempted to replicate Inestrosa’s original findings, she used a mouse behavioral task in which the animal swims to find a submerged platform. But degus don’t naturally swim, Lee says, which muddied her behavioral results.
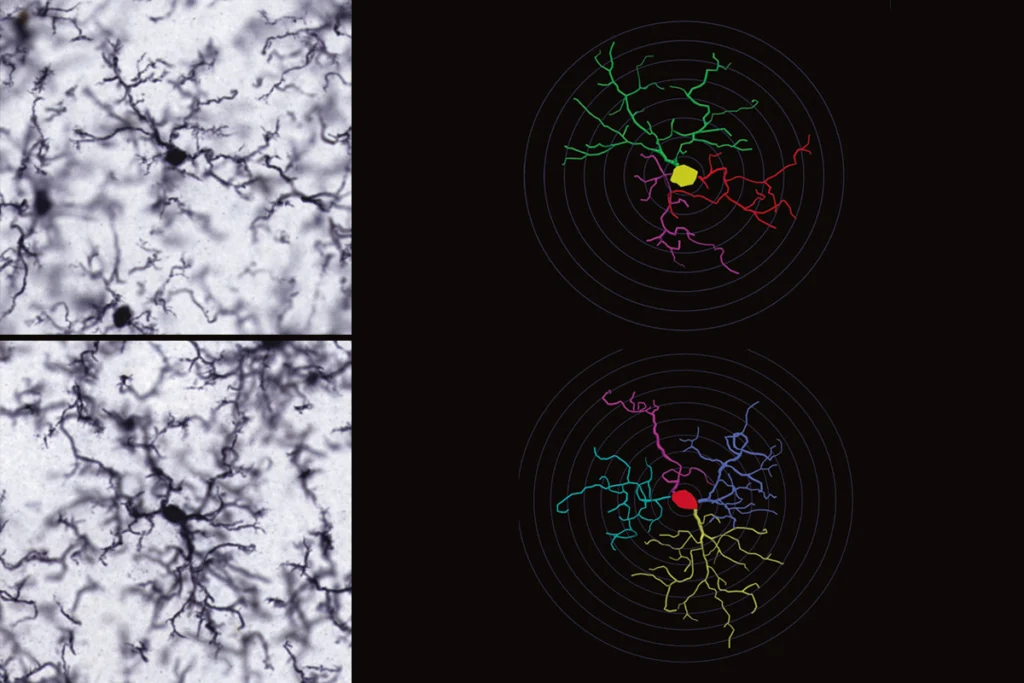
So Deacon developed a test based on a natural degu behavior: burrowing. He filled a plastic cylinder with food pellets and weighed how much food a degu dug out from the tube. After six hours, some older degus had dug out all of the food, Cogram and Deacon reported in a 2015 study. But other aging degus didn’t dig at all.
The latter group—dubbed “poor burrowers” in the study—had plaques and tau tangles in their brain; the “good burrowers” didn’t. The poor burrowers also had greater expression of genes encoding proteins implicated in Alzheimer’s disease in people, amyloid precursor protein and apolipoprotein E (ApoE), and those involved in oxidative stress pathways.