Certain rats housed at the University of Michigan’s Molecular & Behavioral Neuroscience Institute give the researchers who study them two very different greetings. Some animals scurry away from a scientist’s reaching hand, frozen with fright. Others eagerly approach the hand, as if meeting a long-lost friend.
Both groups come from the same stock, but they have been selectively bred to reflect two extremes of temperament: painfully shy versus uninhibited. In people, the former is associated with an “internalizing” coping style — a tendency to ruminate and withdraw from others — and is linked to depression, anxiety and obsessive-compulsive disorder. People with the latter temperament, by contrast, project their feelings outward and are more prone to addiction, conduct disorder and attention-deficit/hyperactivity disorder.
That inner nature is largely inherited, but, according to a flurry of published studies about the differently dispositioned rodents, it can also be changed: Targeting certain inflammation-related cells pharmacologically or adjusting aspects of a rat’s environment — especially during adolescence — can reshape its emotional reactivity and resilience to stress. If the same holds true in people, the findings could lead to a variety of therapeutic avenues — including ways to shift a potentially challenging emotional nature without medication.
It’s impossible to understand any aspect of our lives without accounting for its emotional component, says lead investigator Huda Akil, professor of neurosciences and psychiatry at the University of Michigan. “But scientifically, emotions feel messy. What you find emotionally potent, somebody else might feel neutral about. I think that’s why it wasn’t such an attractive area to study, even though a lot of people are fascinated by it.”
She and her team have spent the past two decades studying animals with extreme responses to their environment to understand why some behaviors become pathological in people. “We don’t have genes for depression; we have genes for how we respond to the world. What predicts future depression are personality features, like impulsiveness,” says Akil, who presented her team’s body of work on the rodents — some of it not yet published — at the 2023 annual meeting of the Society for Neuroscience in Washington, D.C., in November.
Animal work doesn’t always translate to people, but these rats “absolutely” mirror human traits, says Inga Neumann, professor of neurobiology and animal physiology at the University of Regensburg, who studies emotional responses in inbred rodents but is not involved in Akil’s work. Although most people exist somewhere in the middle of the temperamental bell curve, the extreme personalities of selectively bred animals “is where you can reveal substantial differences.” The rodent findings also likely apply to people because the limbic system — a collection of brain structures that regulate emotion — is so well conserved.
The big difference between species, Neumann says, is that people can employ reasoning to process their feelings. “We should never neglect the fact that we have stronger cortical input,” she adds. “Whatever we reveal can be translated to humans, but the real picture is much more complex.”
I
n the late 1990s, Akil and her colleagues began screening rats based on the animals’ responses to novelty — a key predictor of human temperament. Placed in unfamiliar surroundings, those rats displayed all sorts of behavior: Some were eager to explore, others were scared stiff, and many fell somewhere in between. After multiple generations of breeding, however, the team generated rats with responses that are extreme, fixed and highly heritable.Like people, the novelty-averse rats are more likely than novelty-seeking rats to show signs of negative emotions — including anxiety-like behavior, heightened fear responses and a decreased interest in sugar — after experiencing mild stressors, such as temporary water deprivation or a predator’s scent. By contrast, the novelty-seeking rats are more likely to self-administer cocaine and show other impulsive behaviors and addiction-related traits, Akil and her colleagues have shown.
What’s more, the novelty-avoidant rats also show different patterns of gene activation compared with their thrill-seeking counterparts, according to a 2021 meta-analysis by Akil’s team that analyzed 43 generations of rodents. For instance, novelty-seeking rodents show elevated expression of BMP4, which encodes a protein known to suppress neurogenesis. These rats also express less glucocorticoid receptor, a protein thought to act as an initial threat detector, according to other studies by the group.
This receptor may play a critical role in shaping lifelong emotional behavior: Mice engineered to overexpress it before but not after weaning show increased anxiety-like behavior and other changes in their emotional reactivity — traits that are associated with increased activity in a hippocampal-amygdala circuit, according to additional research by Akil’s team.
Several gene expression differences in the hippocampus of Akil’s rats also track with the animals’ behaviors, according to a 2023 preprint. Novelty-averse rats showed stronger expression of genes related to microglia activation — normally part of an inflammatory immune response — and mitochondrial energy production, whereas their braver counterparts tended to express genes with anti-inflammatory functions and roles in neuron development and synapse formation.
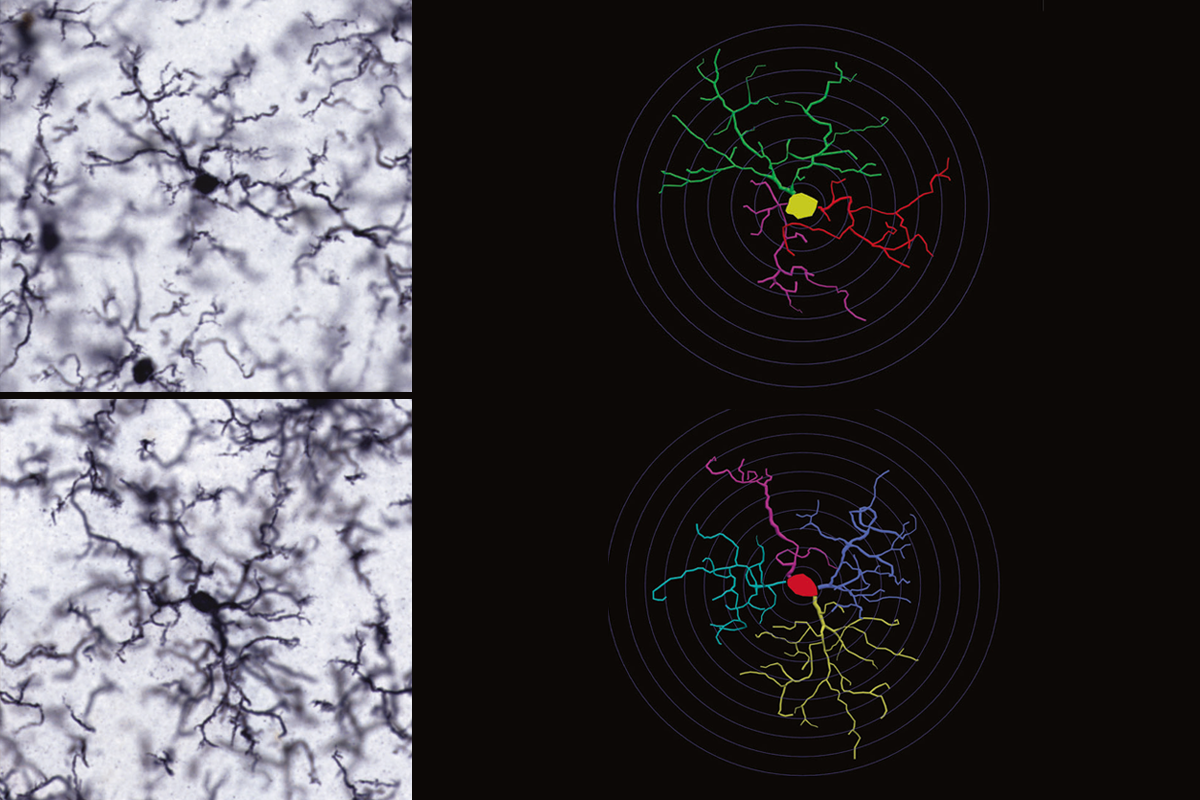
Although the two groups of rats show similar overall numbers of microglia in the hippocampus, microglia in rats that avoid novelty have extra arms and longer branch patterns, and roam larger territories, which corresponds to a state of partial activation known as hyper-ramification.
Hyper-ramification is distinct from the activated state that severe stress or infection trigger in microglia, says Pamela Patterson, assistant research scientist in Akil’s lab. “It’s low-level chronic stress, despite never being exposed to anything stressful,” she says.
Other animal models display similar glial alterations, too. A meta-analysis of five different rodent models of depression revealed increased expression of microglial genes — and reduced transcription of astrocytic genes — in the hippocampus, according to unpublished work from Akil’s lab. And an independent set of rats that Neumann and her team have bred to be highly anxious have fewer microglia in the prefrontal cortex than do controls, suggesting that stress may trigger the cells’ migration to different brain regions.
A
dditional work by Akil’s team and others implicates the brain’s inflammatory response in shaping an animal’s temperament. For example, it’s no surprise that placing typical mice in a cage with an aggressive littermate — a paradigm known as repeated social defeat — triggers anxiety behaviors and the accumulation of immune cells in the brain, as one study found. But mice lacking a receptor that recruits monocytes — the body’s equivalent of microglia — into the brain don’t become anxious. Similarly, when Akil’s novelty-averse rats are treated with minocycline — an antibiotic that inhibits microglia — they appear to be more sociable and less anxious than usual.Despite those findings, whether neuroinflammation causes an anxious disposition is unclear. In most cases, anxiety is probably triggered by other factors, says Jonathan Godbout, professor of neuroscience at Ohio State University, who was involved in the social defeat study. “But if you have activated microglia thrown into the mix, it’s going to make those behaviors more exaggerated,” he says.
Injecting rats with the growth factor FGF2 — what Akil calls “the brain’s natural antidepressant” — also alleviates anxiety-like behaviors in the timid rodents under normal conditions, and in both timid and outgoing rats following a mildly stressful experience, according to unpublished work.
Akil and her colleagues have shown they can boost FGF2 expression in the hippocampus and lower anxiety behaviors in the animals simply by moving them from their standard cage into one filled with toys and obstacle courses, especially in novelty-averse rats. So, too, an enriched home and social life during adolescence can make the animals more resilient to stress in adulthood, a new preprint by the group suggests.
Enhancing the resilience of animals genetically disposed to mood disorders is promising, Patterson says. “It means you’re not stuck in a genetic hole. There are ways out, and that’s encouraging.”
The positive impact of an enriched environment may actually stem from mild stress, she adds. In the same way that moving house can be stressful, being transferred to a new — albeit more exciting — cage might be a little nerve-wracking. Other work shows that adolescent rats subjected to another form of stress — something akin to interrogation tactics — in which they endure a sleepless night on damp bedding, with strobe lights and the scent of a predator, fare better than adult animals in the same situation. And later in life, those adolescent rats also show fewer signs of despair than is typical when forced to swim for long periods, suggesting that the stressful experience in their youth inoculated them against future challenges.
Exactly how mild stress during adolescence exerts its protective effects is unclear but would be interesting to know, Patterson says. “I think about that with my own kids. I look at them and think, what experiences can I give them that will give them that resilience?”