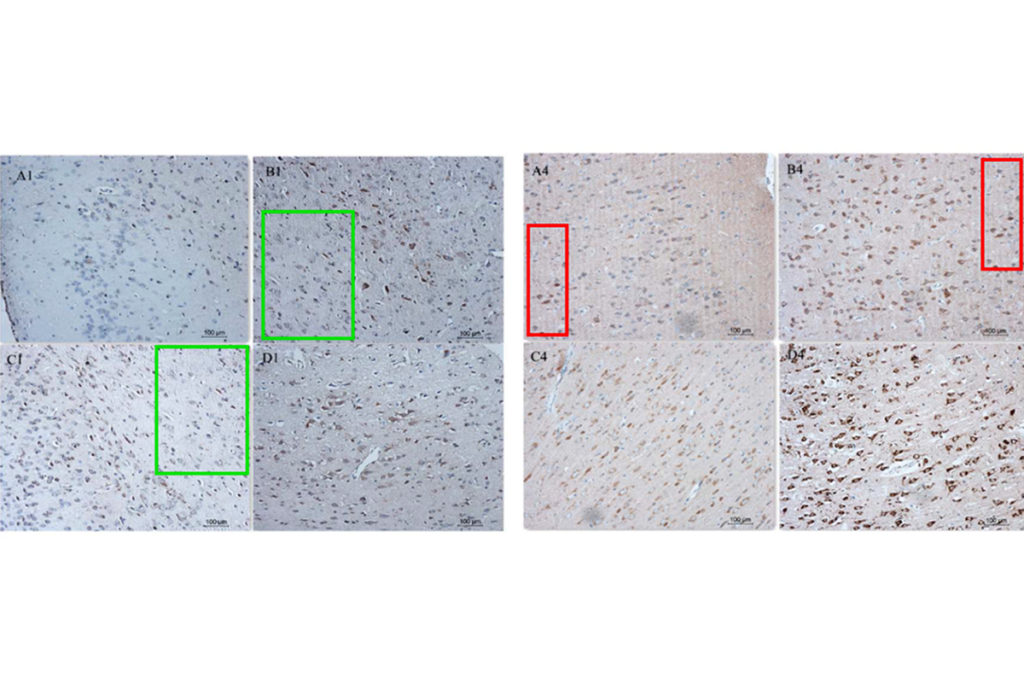
Gary Dunbar, a neuroscientist at Central Michigan University, has had to retract a paper on Alzheimer’s disease while in the midst of trying to replicate its findings.
It’s a painful coda to a years-long saga involving flawed data from a close collaborator, Panchanan Maiti, which has led to two previous retractions and three corrections.
“This has been more than a nightmare,” Dunbar says. “There’s no question that our lead author put in duplicate images.”
The now-retracted paper, published in the International Journal of Molecular Sciences in 2020, suggested that boron-based compounds might protect against Alzheimer’s-like pathology in mice by reducing neuroinflammation and amyloid plaques. It has been cited 24 times, according to Clarivate’s Web of Science platform.
According to the retraction notice published on 3 July 2025, there was overlap in several images used in the paper, which were supposed to be from different experimental conditions. Dunbar and his co-authors were “not able to satisfactorily explain the presence of the above-mentioned irregularities.”
The journal has also added an expression of concern regarding “the integrity of a number of figures” in a second paper from Dunbar’s lab, published in 2021, which suggested that the GLP-1 receptor agonist liraglutide, an antidiabetic drug, has similar properties.
Maiti did not respond to a request for comment sent via Facebook and LinkedIn.
T
he controversy began in October 2021 after suspicions were raised on PubPeer, a post-publication review site, about a string of error-ridden papers Dunbar co-authored with Maiti.Central Michigan University soon launched an investigation, which cleared Dunbar of playing an active role in the serial misconduct after Maiti admitted fault. University officials still chastised Dunbar for failing to perform proper due diligence.
Then, in October 2022, the Office of Research Integrity (ORI) at the U.S. Department of Health and Human Services requested the university open an inquiry into the boron paper, which had been partially funded with a collaborator’s federal grant.
The university submitted its inquiry report about the boron paper to the ORI in January 2023, stating that a formal investigation was not necessary. A university spokesperson responded to a request for comment about the current status of the case to say they are looking into the matter.
In April 2023, Dunbar wrote the journal to request retractions of both the boron and the liraglutide papers. “Although we still have confidence in the in vitro work,” Dunbar wrote, “we were unable to verify the histological data, despite hundreds of hours of additional work. Presently, we are in the process of redoing the in vivo portions of the liraglutide and boron studies, but this will take several months.”
As the editorial board was considering the retraction, Molly Lu, an editor at the journal’s publisher, Switzerland-based MDPI, wrote Dunbar to ask “if there is a possibility that you repeat the experiment and provide original figures?”
“The answer to your question is a definite yes,” Dunbar wrote back in October 2023.
Dunbar was already working on an attempt to replicate both studies and had received approval from his animal care committee for one of them. “I hope you might convince your colleagues to allow us time to redo critical parts of these studies,” Dunbar wrote, “so that we can provide the readers with an accurate and complete analyses, along with access to the complete data sets for both of these studies.”
The experiments, which Dunbar began running in tandem, have taken significantly longer than anticipated, Dunbar says. The journal informed him earlier this year that it was retracting the boron paper without waiting for further results, though it would consider a new submission when the experiments are complete.
The liraglutide paper, meanwhile, remains in limbo. Maiti was not the lead author on that study.
According to the expression of concern published on 8 May, “the Editorial Board has decided to allow the authors to confirm the validity of the overall findings by performing re-experimentation on certain elements of this study.”
Lu did not respond to an emailed request for a comment about why the two studies were treated differently. Liraglutide has shown promise for reducing neuroinflammation in other mouse studies and at least one phase 2 clinical trial in patients with mild Alzheimer’s disease.
Dunbar plans to publish no matter how the new results turn out. “We’re doing the tissue analysis now, so my guess is I’m hoping by the end of August we’ll have everything done and be able to submit right away,” he says.