Treating neurons and brain organoids with a modified form of CRISPR rescues the effects of pathogenic variants in two high-confidence autism-linked genes, according to a new preprint. The approach boosts the expression of the genes, CHD8 and SCN2A, by targeting structures that regulate them.
SCN2A codes for an ion channel that helps propagate electrical signals across the brain. Variants in that gene are associated with seizures, autism and intellectual disability. CHD8 is involved in the remodeling of chromatin, the complex of DNA and proteins that makes up chromosomes. People with faulty copies of CHD8 typically have autism and a larger-than-average head.
The work suggests that the impact of autism-related variants could be reversed by altering gene expression, as opposed to altering genes directly, although more research is needed before the technique can find applications in the clinic, experts say.
“This is another way in which we can regulate genes that are extremely important in [autism], so that’s a major finding,” says Kevin Bender, associate professor of neurology at the University of California, San Francisco, who was not involved in the study. “But it’s really the first step in understanding whether this approach is broadly applicable.”
In the past, Bender and his colleagues used the same version of CRISPR, which activates genes rather than editing them, to boost the expression of SCN2A in mice with a harmful variant in one copy of the gene. The treatment corrected problems in the animals’ neurons.
By increasing the expression of SCN2A in mutant neurons and brain organoids, the new study confirmed many of the previous findings. But the work also revealed, for the first time, that activating gene regulatory elements with CRISPR could counteract the effects associated with harmful variants in CHD8.
Because that gene controls the expression of thousands of other genes, “to see that the upregulation of CHD8 is restorative in some way is really exciting,” Bender says.
T
o increase SCN2A expression, previous work targeted gene promoters—DNA snippets that initiate the transcription of DNA into RNA. Although this approach doesn’t seem to cause harm, dramatically increasing the expression of genes such as CHD8 could lead to deleterious consequences, says study investigator Daniel Geschwind, distinguished professor of neurology, psychiatry and human genetics at the University of California, Los Angeles.So Geschwind and his team used CRISPR to boost activity in noncoding regions of the genome called enhancers, which can regulate a gene’s transcription, in this case linked to SCN2A and CHD8.
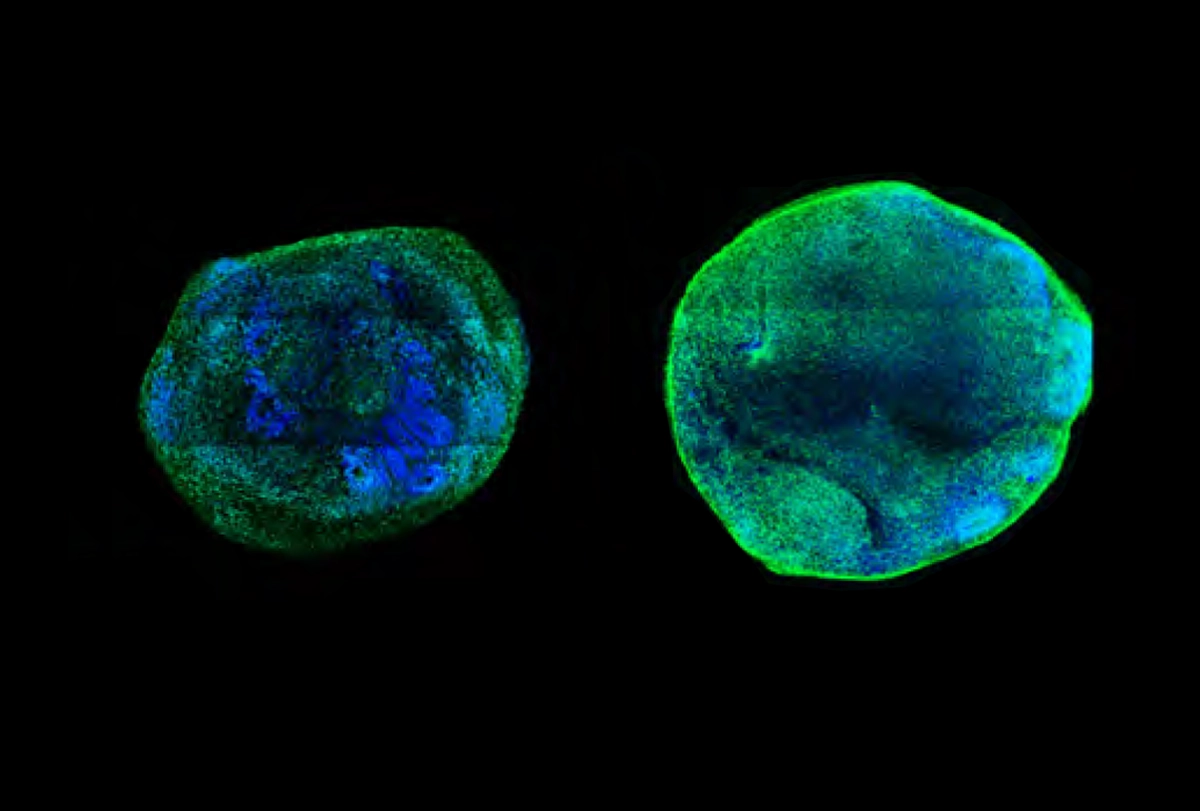
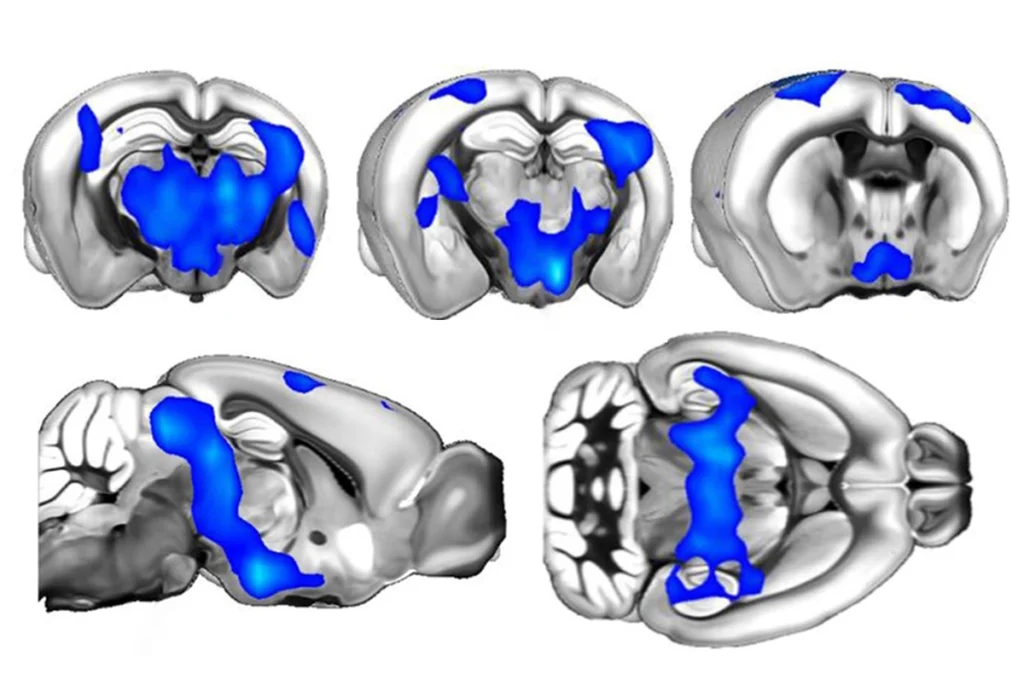
Boosting enhancers of CHD8 in neurons and brain organoids that lacked a functional copy of that gene led to a reduction in organoid size and in the number of differentially expressed genes. This finding suggests the intervention can rescue cells: without it, CHD8 mutant organoids are larger and show an over-proliferation of neural progenitor cells in comparison with controls that have two functional gene copies.
Similarly, enhancing the expression of SCN2A reversed the problems observed in neurons and brain organoids that lack the gene, including impaired development, reduced excitability and sluggish responses to electrical currents.
The team posted their findings on the preprint server bioRxiv in March.
Identifying enhancers for SCN2A and CHD8 was a feat in itself, Bender says. Enhancers aren’t typically located close to the genes they regulate. To find them, scientists must undertake “a treasure hunt,” he says. “Their ability to do that was really remarkable—and they’ve done it for two major [autism-linked] genes.”
Unlike traditional CRISPR approaches, which cut DNA to delete or insert variants and can have off-target effects, CRISPR activation may be less likely to cause harm. But before the approach finds its way to the clinic, researchers will need to determine its safety profile and look for any unintended consequences, says Nadav Ahituv, professor of bioengineering and therapeutic sciences at the University of California, San Francisco, who was not involved in the study.
Ahituv, who has worked with Bender on activating SCN2A using CRISPR, is co-founder of a company that is developing CRISPR therapies that target SCN2A and SCN1A, which has also been linked to autism.
Researchers must also identify a suitable way to deliver the intervention to the brain, Geschwind says. “People are working out in-utero gene delivery, so I don’t think it’s that far off in the next decade,” he says. “I’m fairly optimistic about this type of approach.”
The new study, he adds, also avoids the need to invest significant resources and time into deciphering the functions and mechanisms of individual genes, as well as developing methods to counteract them. Instead, it uses a gene’s regulatory elements to activate its expression. “To me, that’s an exciting therapeutic shortcut.”