This paper changed my life: Sandra Jurado marvels at the first-ever 3D model of a synaptic vesicle
In this 2006 Cell paper, Shigeo Takamori and his colleagues showcased the molecular machinery of synaptic vesicles in outstanding detail. Their work taught me that these aren’t just passive containers for neurotransmitters but dynamic, precision-built nanomachines.
Answers have been edited for length and clarity.
What paper changed your life?
Molecular anatomy of a trafficking organelle. Takamori S., Holt M., Stenius K., Lemke E.A., Grønborg M., Riedel D., Urlaub H., Schenck S., Brügger B., Ringler P., Müller S.A., Rammner B., Gräter F., Hub J.S., De Groot B.L., Mieskes G., Moriyama Y., Klingauf J., Grubmüller H., Heuser J., Wieland F., Jahn R. Cell (2006)
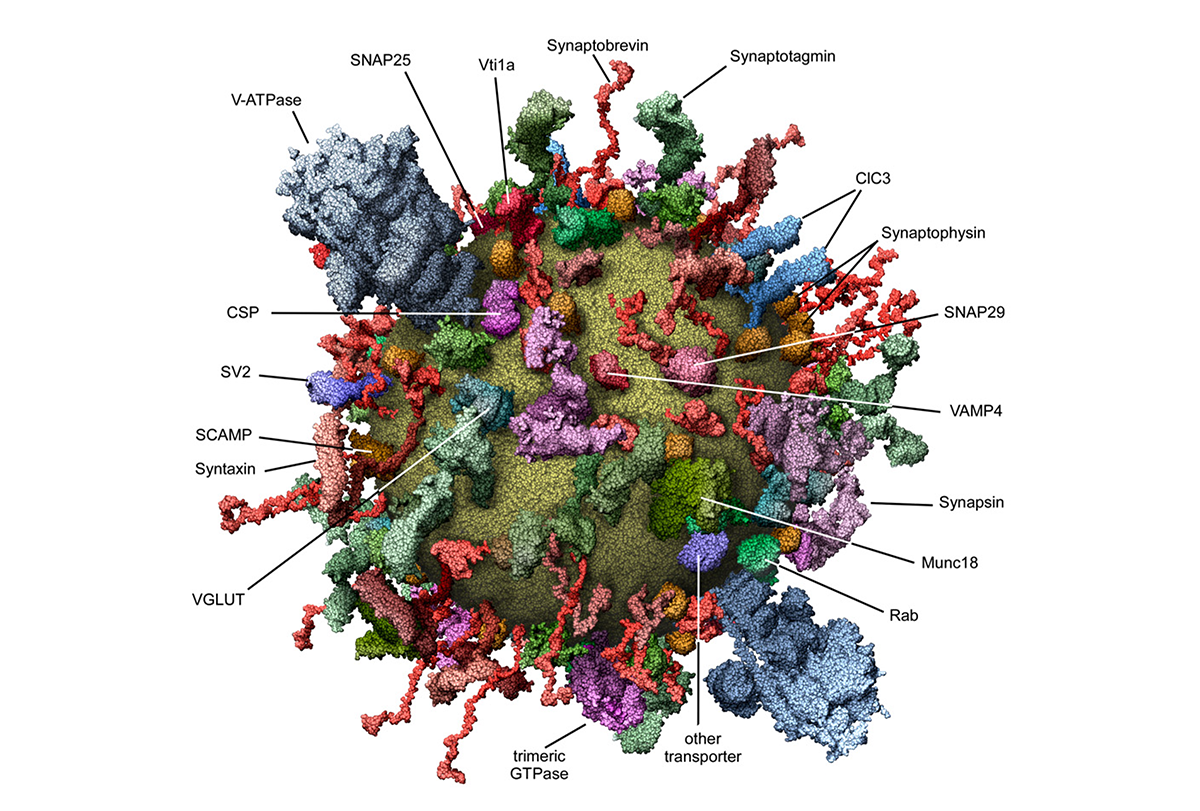
This study presents the first comprehensive molecular description of a trafficking organelle using synaptic vesicles (SVs) as a model. By integrating proteomic, lipidomic and biophysical data, the researchers quantified the copy number of major vesicle proteins, characterized the lipid composition and constructed a 3D molecular model of an average SV.
The findings reveal that SVs are protein-dense organelles with a high diversity of trafficking proteins and contain multiple copies of essential components for neurotransmitter uptake and membrane fusion. Notably, the researchers found only one to two copies per vesicle of V-ATPase, a proton pump protein that establishes proton gradients and drives neurotransmitter uptake, making it an exception among essential SV proteins.
When did you first encounter this paper?
During my postdoctoral work at Stanford University, around 2010, when I was deep in the world of synaptic plasticity and glutamate receptor trafficking. I remember sitting in a corner of the department library, flipping through a stack of aging issues of Cell, when I stumbled across the paper. It stopped me in my tracks.
The reconstructed model of an SV, meticulously packed with all its principal molecular components, was striking. At first, I couldn’t even tell what I was looking at. It resembled a molecular piñata—tiny, spherical and absolutely bursting with surprises inside and out—so densely packed it looked like one tap could trigger an exocytic meltdown. I was captivated.
Why is this paper meaningful to you?
What struck me wasn’t only the level of detail but the audacity of it. The authors didn’t just characterize a few components; they mapped out an entire SV, molecule by molecule. It clearly showed how unbelievably crowded and intentionally organized these vesicles are, which is what makes that figure both scientifically astounding and oddly funny when you realize how much nature managed to cram into roughly 40 nanometers. They created a quantitative, structural model of this tiny organelle, transforming it from a black box into a living, breathing machine.
At the time, my research was focused on receptor trafficking in the context of synaptic transmission and plasticity. In that framework, the cargo—neurotransmitters and neuropeptides—and the receptors took center stage, and the vesicles themselves were mostly an afterthought.
But this paper made me see the vesicles themselves. They weren’t just passive couriers—they were dynamic, precision-built platforms. That shift in perspective sparked a broader interest in release mechanisms, especially those governing neuropeptides such as oxytocin, which are secreted through unconventional routes—for example, somatodendritic exocytosis. Unlike classical synaptic release, this mode resembled the receptor trafficking I had been studying, but with the added intrigue of influencing a wide range of fundamental homeostatic functions. This paper became a foundation stone for the questions I would begin to ask next.
How did this research change how you think about neuroscience or challenge your previous assumptions?
Until then, I had thought of the sequence of events during synaptic vesicle release—docking, priming, fusion—in relatively abstract terms. This paper challenged me to consider the vesicle itself as a central player, densely packed with proteins, architecturally constrained and designed for a specific functional purpose. Even something as subtle as the copy number of a single V-ATPase subunit could shift its physiology. That level of molecular precision made me rethink everything I thought I knew about secretion, how much I had overlooked and how much there still was left to learn.
How did this research influence your career path?
Reading this paper didn’t just expand my understanding; it quietly reoriented my career. It gave me a new language and conceptual framework to explore oxytocin release and inspired me to pursue the question of how vesicle architecture might encode different signaling modes, particularly those related to homeostasis and complex functions such as social behavior. Even though it has been almost 20 years since it was first published, the 3D reconstruction shown in figure 4 is still my go-to image to show the complexity of SVs. It still has the same wow effect on me as it did the first time I saw it.
Is there an underappreciated aspect of this paper you think other neuroscientists should know about?
One aspect of this paper that I think remains underappreciated is the way it frames the vesicle not as a passive container but as an active, tightly packed nanomachine. In addition to emphasizing the heterogeneity of the vesicle proteome, the paper highlights that, alongside a core set of “genuine” vesicle residents, there are many “visitor” proteins that associate with the SV only during specific stages of their life cycle. The idea that function is encoded directly into structure at such a granular level shapes how I think about cellular mechanisms and continues to fuel my curiosity to this day.
Recommended reading
This paper changed my life: Ishmail Abdus-Saboor on balancing the study of pain and pleasure
This paper changed my life: John Tuthill reflects on the subjectivity of selfhood
The best of ‘this paper changed my life’ in 2025
Explore more from The Transmitter