Repeated exposure to cocaine and morphine subverts the reward-system neurons that underlie hunger and thirst, according to a new study in mice.
“The nerve cells get scrambled at the neural level in terms of their responses to food and water,” says lead investigator Eric Nestler, professor of neuroscience at the Icahn School of Medicine at Mount Sinai. “So the ability of the brain, in a way, to compute that the individual is hungry or thirsty becomes lost.”
In addiction research, there has been a “long-appreciated hypothesis that drugs of abuse hijack the natural reward circuitry of the brain,” says Marcelo Wood, professor of neurobiology and behavior at the University of California, Irvine, who was not involved in the new work. “It’s something that everyone talks about and writes about,” he says, but the exact physiology behind it “remained rather unknown.”
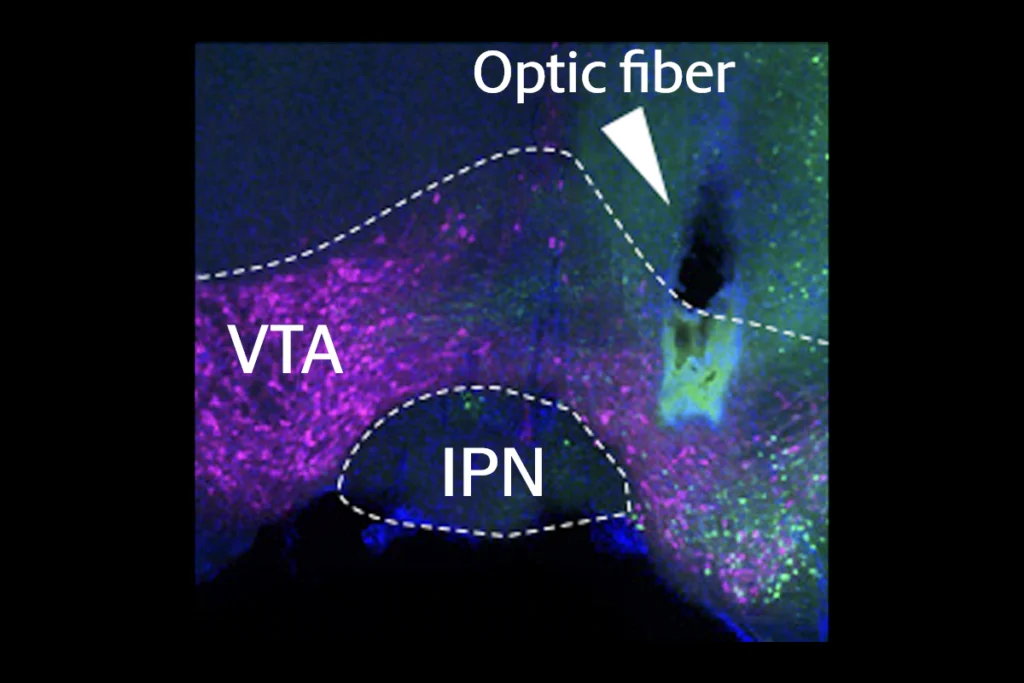
In the new work, mice injected daily with morphine or cocaine for up to five days showed progressively increased activity in neurons in the nucleus accumbens that also respond to food and water, according to measures of FOS protein, a marker of neuronal activation. The drugs also elicited a stronger response than the natural rewards, two-photon calcium images showed, confirming what scientists have thought based on behavioral evidence, Nestler says.
These alterations ultimately curbed the animals’ urge for sustenance, the study also shows: The mice ate less food and drank less water than mice given a saline solution, and lost weight—even after withdrawing from the drugs for three days.
That confirms the hijacking hypothesis “pretty convincingly,” Wood says. “I thought that was brilliant.”
O
nly 4.5 percent of D1 medium spiny neurons (MSN)—named after the “type 1” dopamine receptor they express—were activated by cocaine but not by food or water; about 6 percent were activated only by morphine.“That suggests there’s a massive overlap in response to drug or natural reward, and indicates how drugs hijack natural reward circuitry,” Wood says.
In D2 MSNs, which express the “type 2” dopamine receptor, the overlap was smaller with morphine but not with cocaine, activating about 35 percent not activated by food or water.
After three days without drugs, the D1 MSNs in mice withdrawing from cocaine showed less activity than usual during eating or drinking, whereas in mice withdrawing from morphine, D2 MSN activity was increased and D1 activity was unaffected.
Neuronal activity changes during eating or drinking correlate strongly with the expression of the RHEB gene, according to gene-expression maps from the Allen Brain Atlas. RHEB encodes an enzyme known to regulate the mTOR pathway, which orchestrates the growth of neurons and synapses. Mice that had RHEB knocked out of their nucleus accumbens did not reduce their intake of food or water after drug exposure, the new work also showed. The results were published 19 April in Science.
The findings suggest that RHEB mediates this behavioral change, but it is unclear whether drug experience alters RHEB levels or if the gene is selectively expressed only in the neurons that were active, says Bruce Hope, chief of the Neuronal Ensembles in Drug Addiction Section at the National Institute on Drug Abuse within the U.S. National Institutes of Health. He was not involved in the new work.
Future studies would need to show that cocaine or morphine alter the expression of RHEB before being able to claim that RHEB is responsible, Hope says, and Nestler agrees.
A
nother limitation, noted by Nestler’s team and other scientists, is that the mice were injected with drugs. Follow-up studies should allow animals to access the drugs by pressing a lever or poking their nose in a hole, because “self-administration captures aspects that are more similar to human drug use,” Wood says.Nestler says that he and his team hope to investigate the effects of longer-term exposures to drugs, as well as effects in other key brain areas. They also want to understand how epigenetic mechanisms—for instance, changes to chemical tags on DNA or to histones, the spool-like proteins around which DNA is wrapped in the nucleus—might regulate RHEB.
Histones change in the D1 MSNs of mice given a dose of cocaine, Nestler’s lab reported in a preprint posted on bioRxiv in 2022. This alteration makes a certain region of the chromatin in the cells’ nuclei more accessible—not only while cocaine is in the animals’ systems, but even after 30 days of withdrawal. A second dose of cocaine at that point, imitating a relapse in a person with addiction, further boosts the accessibility of genes involved in, for example, synaptic plasticity and signaling across the synapse, which are targets of transcription factors that cocaine activates.
These changes “are making the brain more vulnerable to the drugs over a long period of time,” Nestler says, which could explain why people with a cocaine addiction can relapse rapidly even after a long period of abstinence.
For the work in the preprint, the team could not see how D1 neuron activity changed over time. The new work provides a clue, but the team still needs to link the epigenetic changes with the physiological ones, which are usually studied separately, Nestler says.
“One of the major needs in neuroscience is to integrate across all these levels of analysis, [which is] really hard to do in the same paper and in one lab,” Nestler says.
The new study required the collaboration of three labs to “take a broader, more complete view of these neural phenomena,” he adds. “I think it does provide a template of what is now possible in the field of neuroscience.”