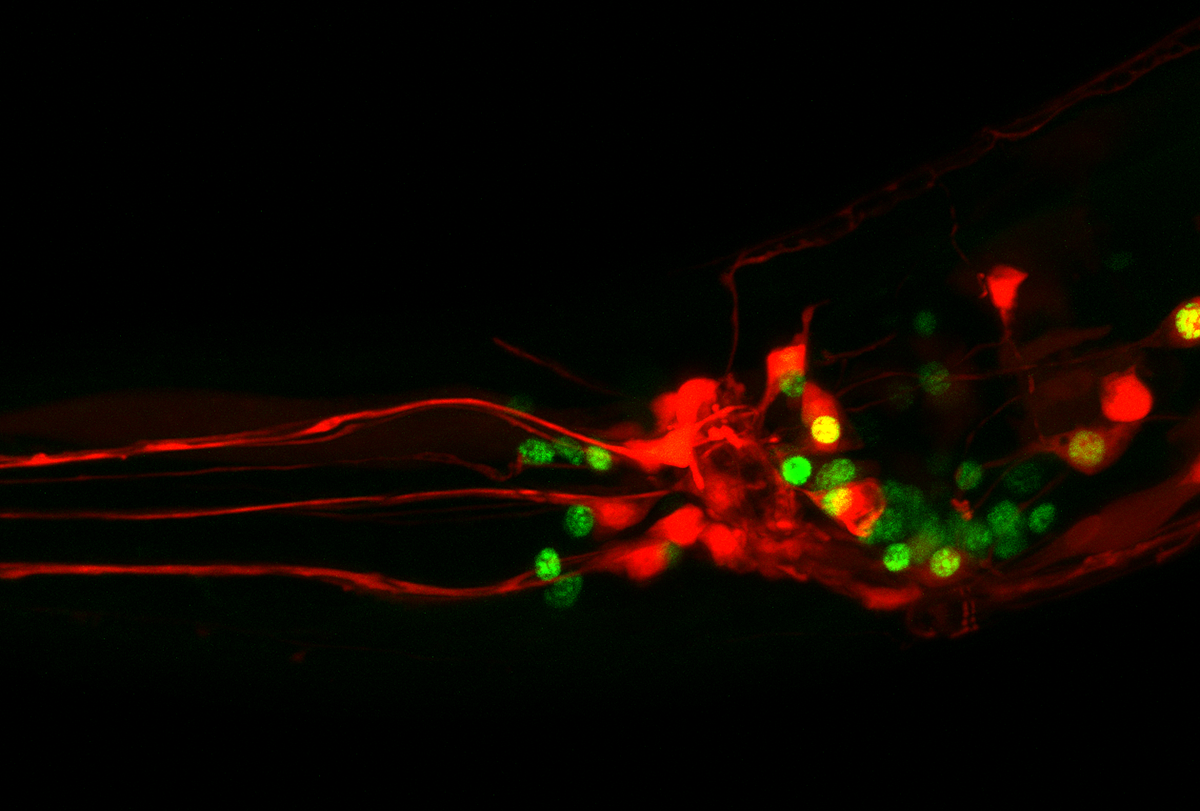
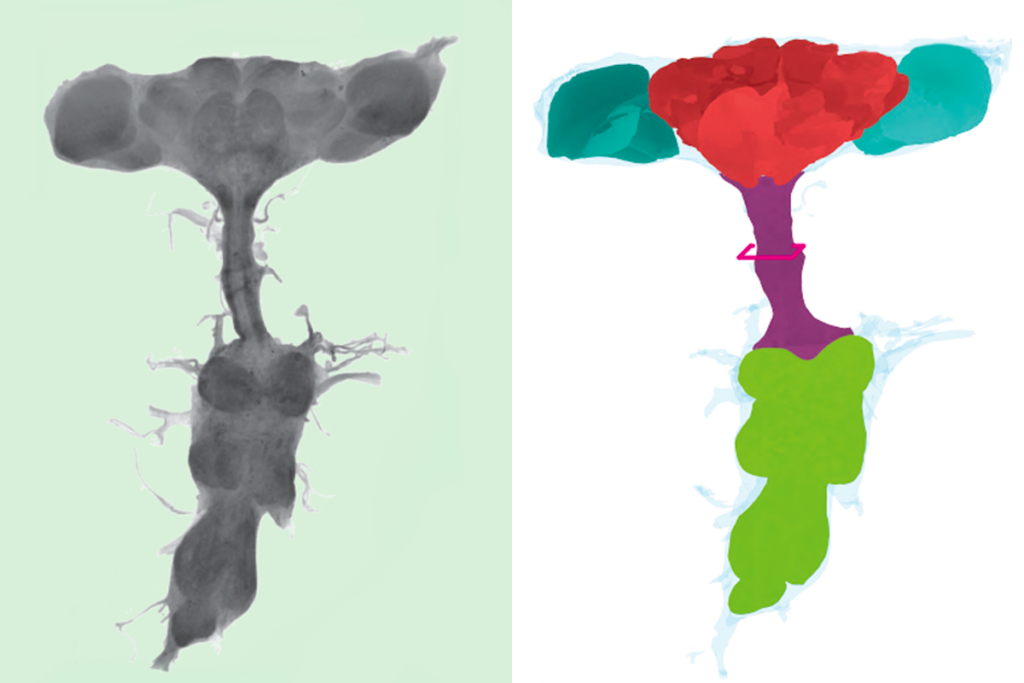
Scientists have had access to maps of synaptic connections within the roundworm Caenorhabditis elegans for decades, yet they have struggled to explain how the organism’s neurons interact to beget behaviors. One reason, critics of the synaptic connectome say, is that the map lacks information about small signaling proteins called neuropeptides, which are released from neurons via vesicles and enable the cells to communicate outside of synapses.
Two new connectomes unveiled last month take this invisible, or wireless, form of communication into account. One depicts where each neuropeptide and its matching receptor reside in the roundworm’s 302 neurons, revealing a neuropeptide connectome that is far denser and more complex than that of its synaptic cousin. The other map traces the propagation of signals among 186 of the worm’s 188 head neurons and shows that the paths sometimes wander outside the lines of synapses.
The new maps are the “most comprehensive to date, grappling with the interactions of synaptic networks and [the] peptidergic network,” says Stephen Smith, science investigator emeritus at the Allen Institute for Brain Science in Seattle, Washington, who was not involved in either study.
Neuropeptides were previously thought to diffuse widely and slowly to tune the excitability of neuronal populations or modulate neurotransmitter release. But the new studies show that neuropeptides form a dense signaling network among neurons and can function like neurotransmitters.
“The idea that the neuropeptide signaling network is just helping the synaptic network to do its job — that I think we are now realizing is not a fully accurate view,” says Isabel Beets, assistant professor of biology at KU Leuven in Belgium and co-lead investigator on the neuropeptidergic connectome work.
B
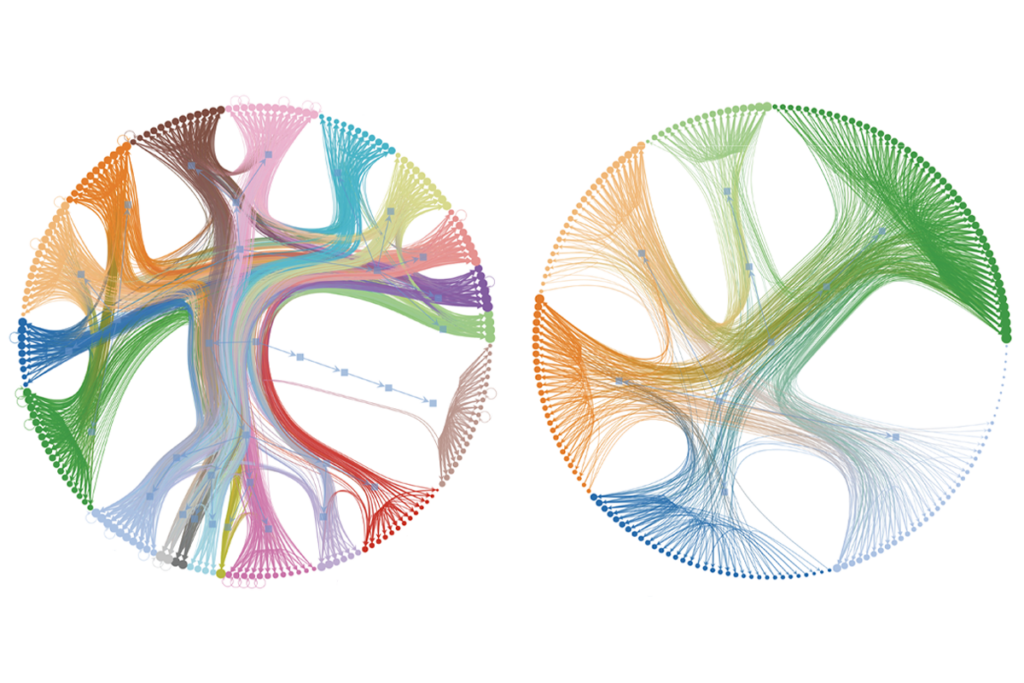
eets and her colleagues created the neuropeptidergic connectome using data from the complete gene-expression profile of the roundworm, along with knowledge of the binding patterns between the organism’s 344 neuropeptides and 161 neuropeptide receptors.Some of the most well-connected neurons are different from those identified by the synaptic connectome, further analysis of the network shows. “They’re not the usual suspects,” says co-lead investigator Petra Vértes, professor of systems and computational neuroscience at Cambridge University in the United Kingdom. “We don’t really know why they’re so important.”
The neuropeptidergic network also has a greater proportion of well-connected neurons compared with the synaptic one, leading the team to describe it as “decentralized.” And all roundworm neurons sport more neuropeptidergic connections than synaptic connections, on average, the researchers found. The findings appeared 6 November in Neuron.
Initially, the sheer density of neuropeptide connections resembled “a big hairball,” says co-lead lead investigator William Schafer, professor of molecular biology at the MRC Laboratory of Molecular Biology in the U.K. By teasing apart that hairball, they identified four main clusters of neurons: hub, sensory, motor and periphery. Sensory and motor neurons link to each other only through hub neurons, the team found, among other discoveries.
“Hopefully, just the principles about how this network is organized will help when people try to map these networks in bigger brains,” Schafer says.
Before this connectome was complete, the redundant functions of neuropeptides frustrated researchers, says Larry Zweifel, professor of psychiatry and behavioral sciences at the University of Washington in Seattle, who was not involved in either new study. Experiments designed to establish the role of a certain neuropeptide by removing it from the organism might result in “literally nothing,” he says. Now, future studies can examine the versatility of neuropeptides and explore why many perform overlapping functions in the brain.
A
synaptic connectome is often viewed as a road map of all possible routes of information flow among neurons in the brain. But just like a road map fails to predict which path cars take and how quickly they travel, a synaptic connectome provides no information about the routes that signals take through the brain, or the strength and nature of neuronal linkages.The second new map begins to address these shortcomings for 186 neurons in the roundworm’s head. It shows the synaptic strength of connections between these neurons, whether they are excitatory or inhibitory in nature, the paths signals take and how quickly they propagate.
The team parsed these molecular details in 113 roundworms by using optogenetics to activate, one by one, 186 of the head neurons with light. They also used calcium imaging to simultaneously record other neurons’ responses across the brain.
Repeatedly testing 23,433 pairs of head neurons, or 66 percent of all possible pairs of head neurons, revealed that just 1,310 pairs, or 6 percent, are functionally connected. Among those sharing a synapse, roughly half are functionally connected.
Anatomy alone failed to predict where the signals would propagate — implying that functional connections form outside of synapses. Mutant animals whose neurons could not release dense core vesicles, and thus most neuropeptides, formed 53 fewer pairs of functional connections than their wildtype counterparts, suggesting that those pairs of head neurons most likely connect via neuropeptides outside of synapses. The findings were published 1 November in Nature.
“A lot of the information that isn’t in the wiring is still really important for predicting the details of the neural signal,” says lead investigator Andrew Leifer, professor of neuroscience at Princeton University.
Also important is the speed of the wireless signaling: These neuropeptides help propagate the signal at a similar speed to neurotransmitters, within one second of optical stimulation, the work shows. Previous studies have shown that neuropeptides can take between tens of milliseconds to minutes to act.
The functional atlas complements the neuropeptide connectome, says Hannes Buelow, professor of neuroscience at Albert Einstein College of Medicine in New York City, who was not involved in either study. Using the functional atlas as a guide, C. elegans researchers can pinpoint the neuropeptide and receptor pairs that fill the gaps in the wired connectome, he says.
“These two stories, while maybe on the surface seemingly different, are actually quite related and complementary, and they — on some level — show very similar things in different ways,” Buelow says.