Adult human cortex does not reorganize after amputation
The results from a new longitudinal study contradict classic findings in monkeys but may not warrant a rewriting of the textbooks just yet.
The adult cortex can rewire itself after injury, according to a series of classic experiments. When a monkey loses sensory input from a finger, for example, the region of the somatosensory cortex dedicated to that finger becomes overrun by inputs from the animal’s nearby fingers or face; the cortical map for the unused finger fades, and nearby maps of other body parts expand.
“This is what I read in my textbook. This is what the lecturers told me in my lectures in university,” says Tamar Makin, professor of cognitive neuroscience at the University of Cambridge.
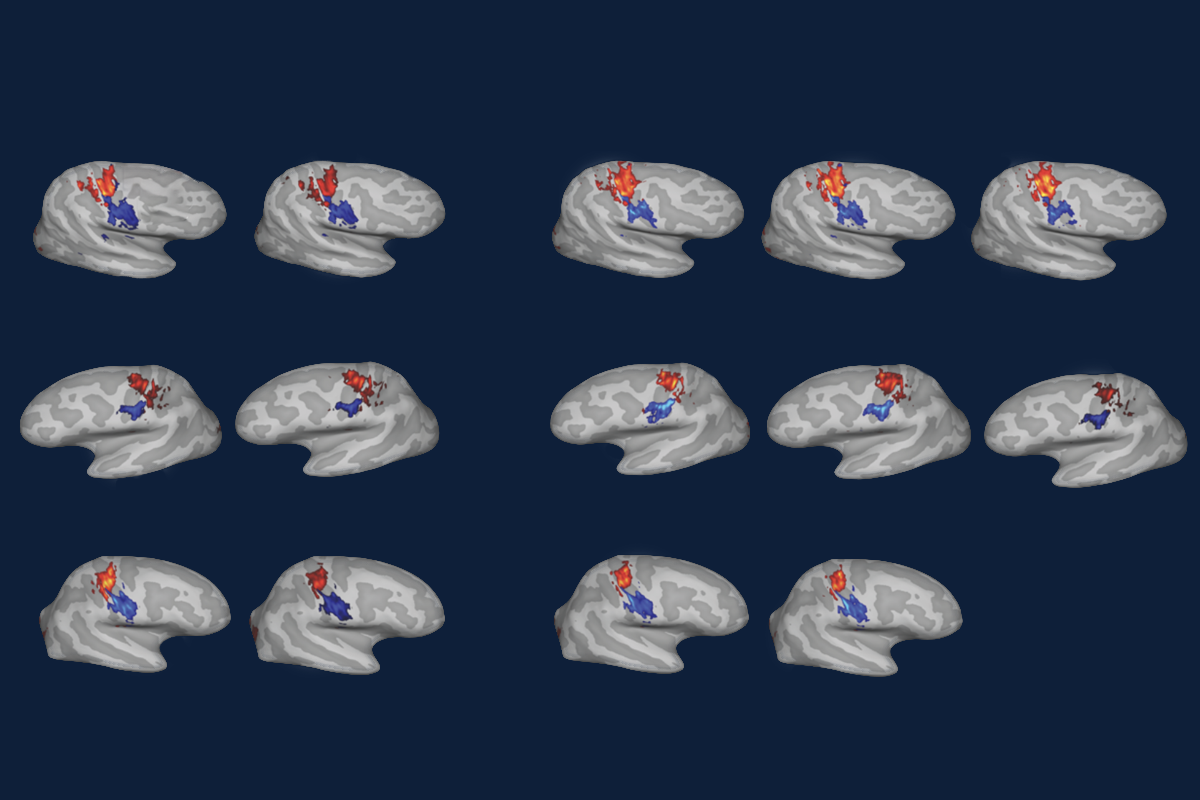
But—contrary to those classic findings—such large-scale cortical reorganization did not happen in three people who lost an arm, according to a new functional imaging study Makin and her colleagues published today in Nature Neuroscience. Instead, the somatosensory map of each person’s hands, feet and lips, generated when they moved or attempted to move that body part, remained stable in the years before and after their hand was removed.
“The representation of the hand persists,” says Makin, who led the study.
The work is the first longitudinal look at whether amputation changes that cortical mapping. The results confirm what previous cross-sectional studies have hinted at, and they should put an end to the debate about how readily the adult cortex can shift its function, Makin says.
But not everyone agrees. The study is an important contribution to the field, and it shows that maps of somatosensation driven by motor input remain stable after amputation, says Ben Godde, professor of neuroscience at Constructor University, who was not involved in the new work or the classic experiments. But that does not mean that other cortical maps are not shifting as a result of changing inputs, he says. “It’s not evidence that there’s no plasticity.”
Still, past studies defined “cortical reorganization” as a repurposing of the cortex after a change in input—something that the new study does not find, says John Krakauer, professor of neurology, neuroscience and physical medicine and rehabilitation at Johns Hopkins University, who was not involved in the work but has previously collaborated with Makin. “Claims were made that you get takeover of function. And there’s just no such thing.”
I
n a series of experiments that began seven years ago, Makin and her colleagues imaged the brains of three people who were planning to undergo an arm amputation to address medical conditions. They asked the participants to move their fingers, toes and lips in the scanner and then used that activity to identify how somatosensory information from each of those body parts mapped onto the cortex.The team repeated the experiment at three and six months after surgery for all three participants, this time asking participants to attempt to move or imagine moving the fingers from the hand that had been amputated. They also scanned one participant a year and a half after surgery, and another participant five years after. With each scan, the resulting maps of the somatosensory cortex remained roughly the same, even at the single-voxel level, the researchers found. Finger-specific activity also remained just as selective before and after amputation. And control participants who did not undergo surgery showed similarly stable maps over time, the team reported.
“This neural configuration before amputation remains after the amputation. And it’s not ‘use it or lose it,’” Krakauer says.
The discrepancies between these findings and those from the classic monkey studies may come down to differences in methodology, says study investigator Hunter Schone, a postdoctoral associate at the University of Pittsburgh.
In the monkey experiments, the researchers identified the animals’ somatosensory maps by recording from neurons while touching the animal’s remaining fingers and face with a glass probe after amputation of a finger. They assigned cortical territory to the finger that elicited the greatest neuronal response, but because the researchers could not touch the missing finger, that digit was not assigned any territory, Schone says. That does not mean that the monkeys’ brains forged new connections or that neurons changed their tuning because of amputation, just that the region was already residually responsive to the adjacent fingers, he says. “You’re unmasking the adjacent digits, which you now assign to that territory—but only because of your method.”
The same goes for a 2021 study that identified changes in a mouse’s somatosensory map for its whiskers after one whisker is removed, Krakauer says. “There were neurons that were always responsive to that whisker,” he says. “What they couldn’t find was a neuron that used to be for whisker A and then now became for whisker B.”
G
odde agrees that a loss of sensory input leads to an unmasking of preexisting connections. But that inevitably leads to changes in how neurons respond to a given stimulus, which is a form of functional reorganization of the cortex, he adds. It is possible that the participants in the new study—people who had limb injuries that required amputation—had already experienced some loss of sensory input that resulted in such reorganization before the study took place, he says.And because the experiments did not directly replicate the methods used in the monkey studies, it remains unclear if a person’s sensory maps driven by touch would also change as a result of a loss of input, Godde says. When the team asked people to move or imagine moving their fingers, toes and lips, those cortical maps were driven by top-down motor inputs, he says. “They compare different things and say the map has not changed.”
Makin says that because movement of the lips activates bottom-up mechanical receptors, that criticism does not hold. “We can absolutely map out the boundaries of the lips, and the lip representation doesn’t shift or change,” she says. So in theory, the challenges are correct, but “in practice, they’re wrong.”
The new work rejects the idea that any “functional remapping”—which would require a shift in neurons’ topographic maps—is happening, Makin says. With their experimental paradigm, the team cannot rule out the possibility that other aspects of the representation have shifted, Makin says. “But maybe now that, I hope, we have a resonating answer to this question, we can close this chapter and start another chapter.”
One potential new chapter is how to treat phantom limb pain, which people frequently experience after amputation, Schone says. Because some research suggests that such pain stems from inappropriate cortical reorganization, treatments attempt to undo that improper mapping. But the new work suggests that is futile, Schone says. Other treatments, such as those that address the peripheral nerves left over after amputation, may prove more successful, he says.
The findings also raise important questions for neuroscientists, Krakauer says. “Something has happened that means that the configuration, the internal structure of that region, has locked into an identity—and it’s not going to change just because it’s no longer getting its original input,” he says. “What is the biology of that resilient hanging-on to your cortical identity?”
Recommended reading
To understand the brain as a network organ, we must image cortical layers

What kinds of support do early-career researchers need?
Explore more from The Transmitter
Body state, sensory signals commingle in mouse whisker cortex
Expanded view of hippocampal function comes into focus

