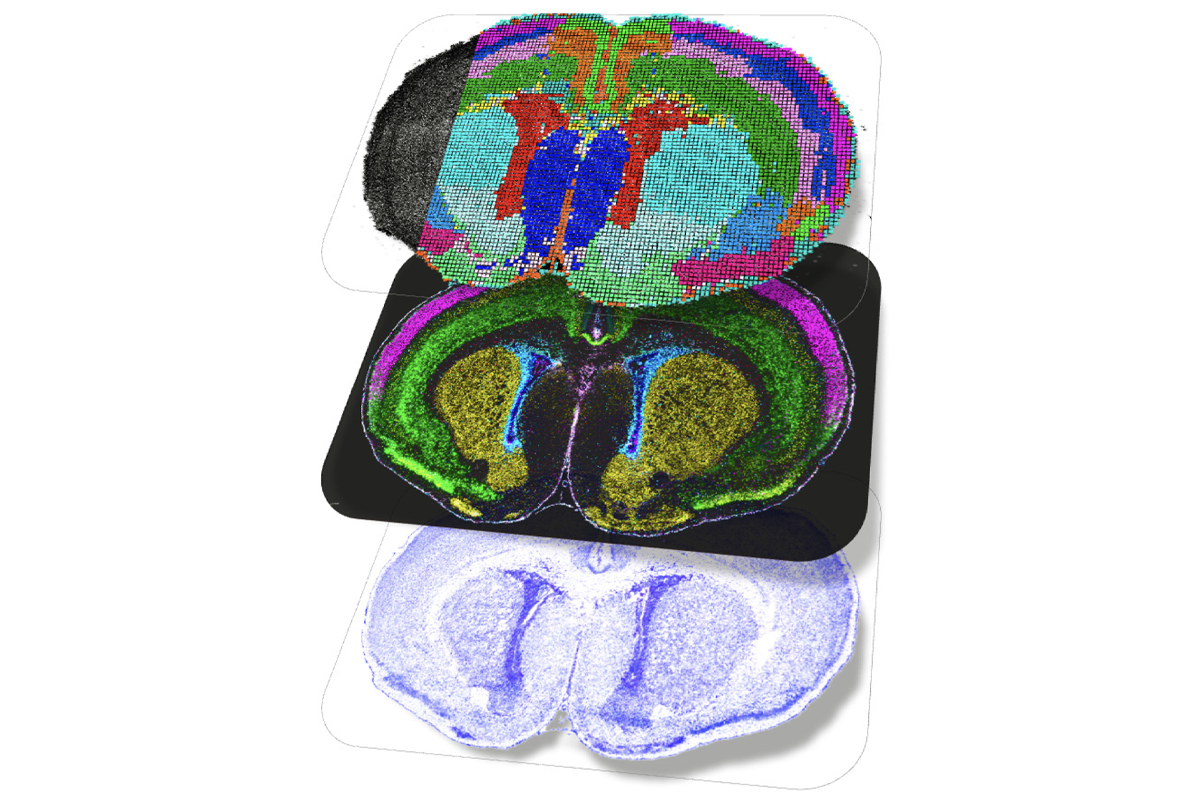
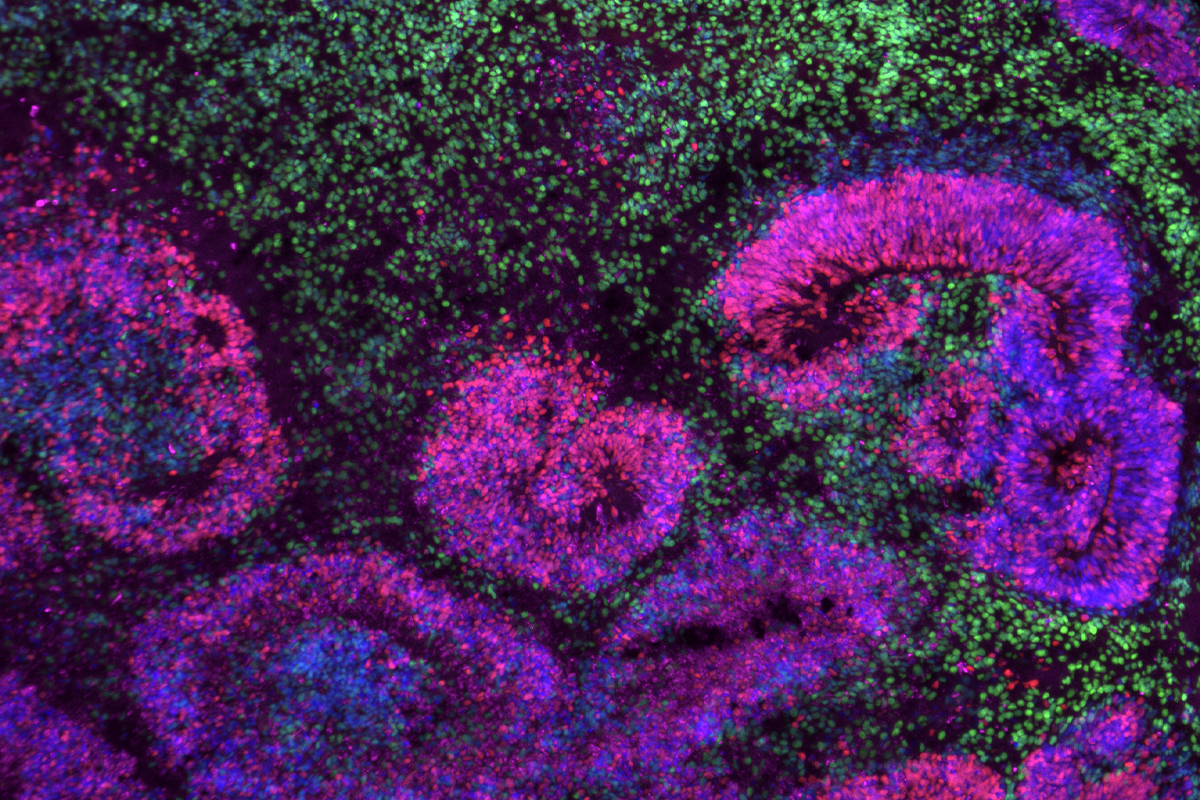
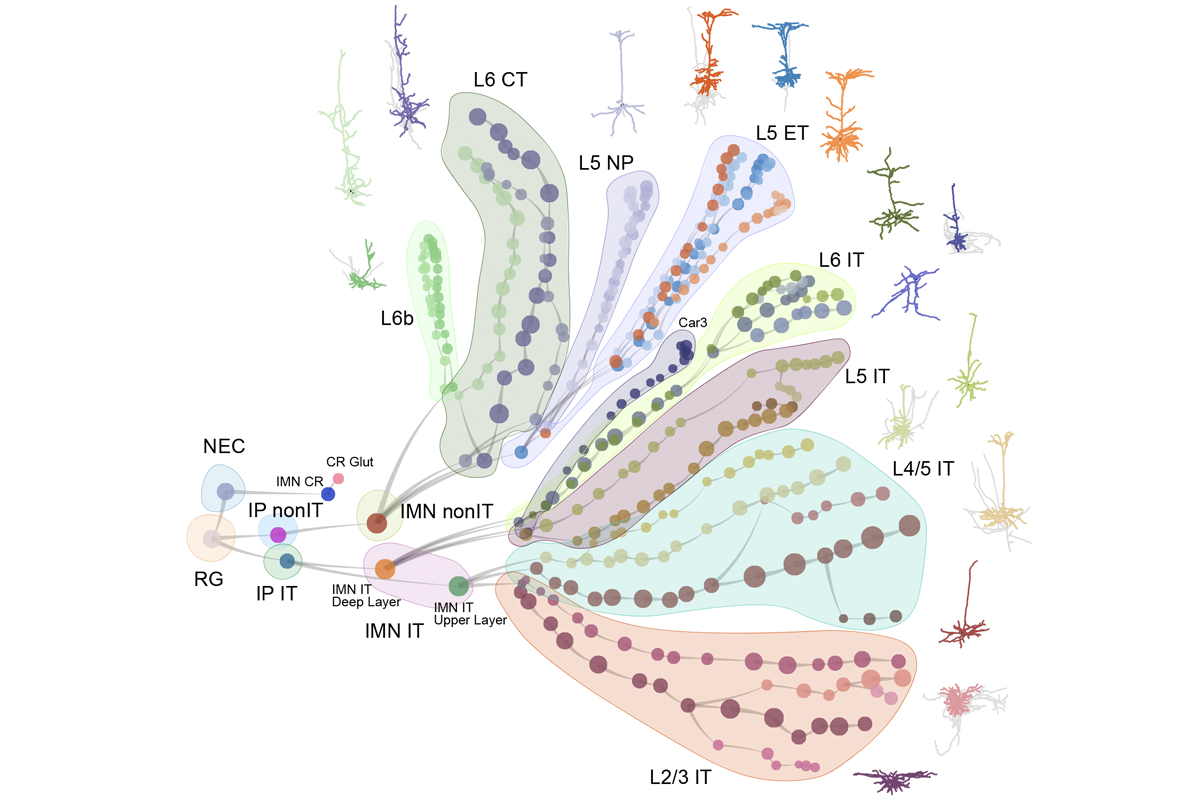
Multiple mouse and human brain atlases track the emergence of distinct cell types during development and uncover some of the pathways that decide a cell’s fate. The findings were published today in a collection of Nature papers.
The papers highlight the timing and location of cell diversification and offer fresh insights into the evolution of those cells. Neuronal subtypes emerge at starkly different times in distinct brain regions, according to multiple mouse studies. And the work upends ideas about cell migration, including the notion that a portion of cortical neurons are made on site, developmental maps of the human brain suggest.
“This is a dramatic revision of the fundamental principles that we thought were true in the cerebral cortex,” says Tomasz Nowakowski, associate professor of neurological surgery, anatomy and psychiatry, and of behavioral sciences, at the University of California, San Francisco and an investigator on one of the new studies.
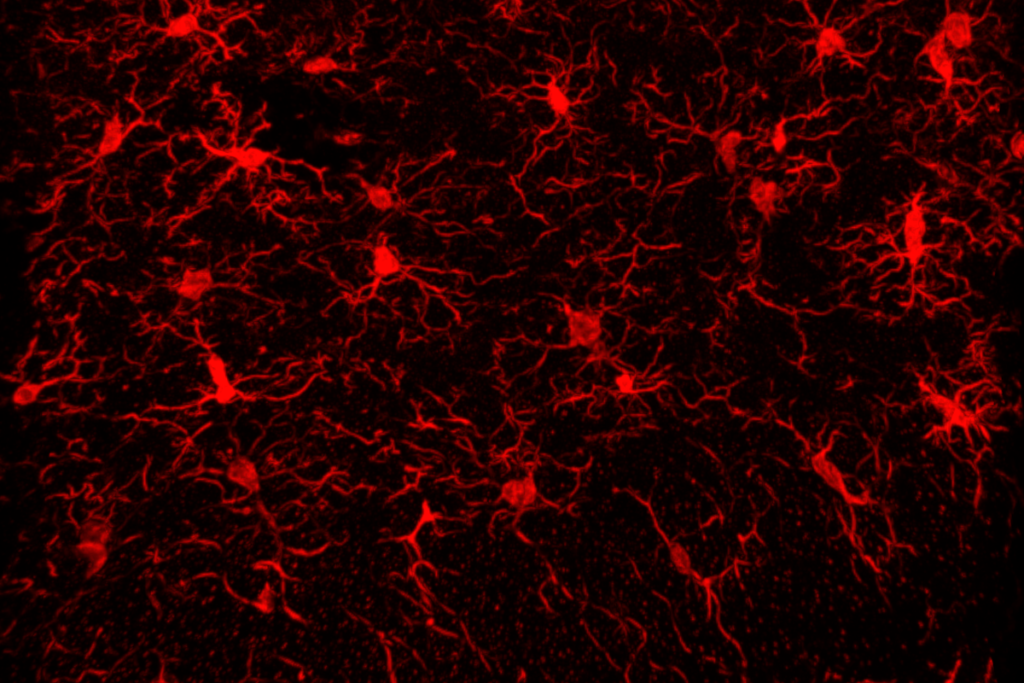
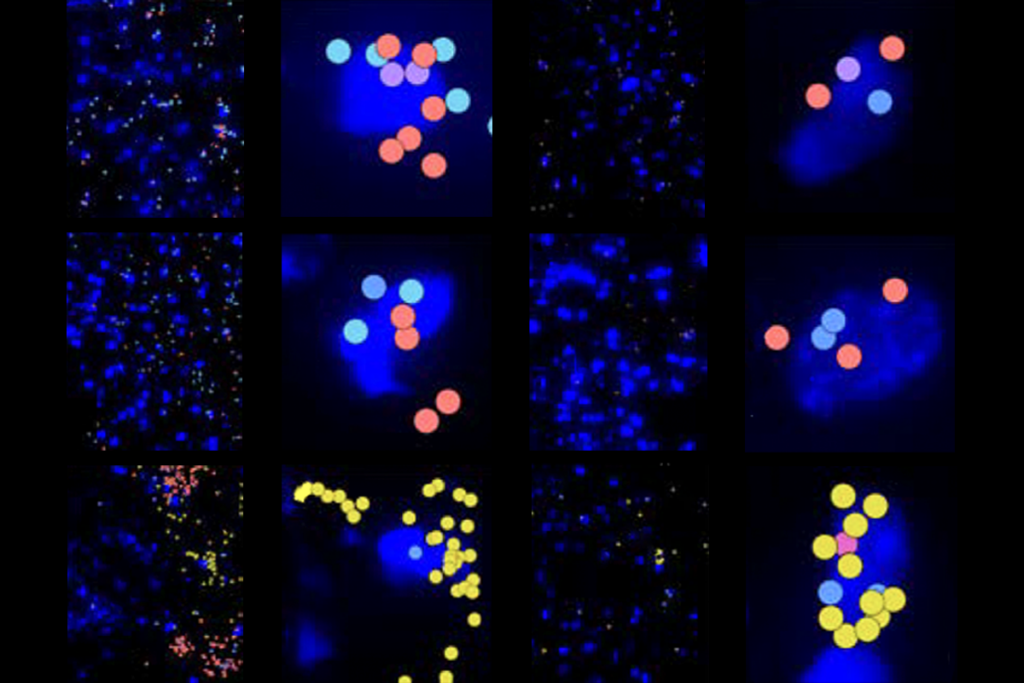
The special issue comprises 12 papers—including 6 newly published ones—from groups working as part of the BRAIN Initiative Cell Atlas Network. The work builds on the network’s complete cell census, published in 2023, that cataloged 34 classes and 5,322 unique cell types in the adult mouse brain.
“Those cell types don’t appear out of a vacuum at the same time,” says Nowakowski, who co-authored a commentary on the new collection. Pinpointing when those cells emerge and where they originate from was the “obvious next question,” he says.
A
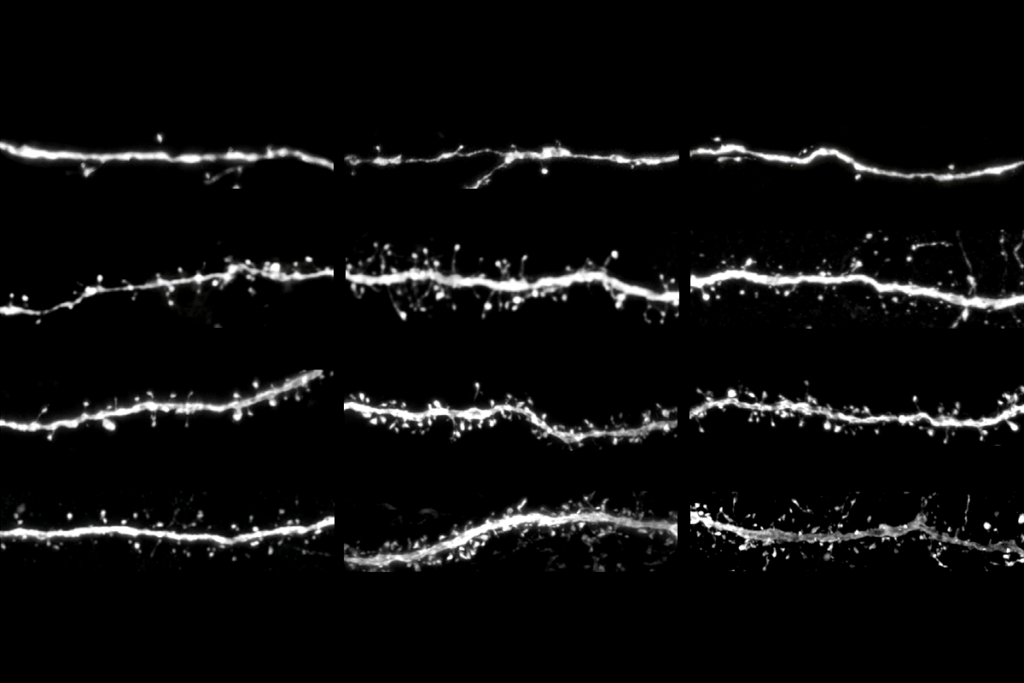
t birth, the mouse brain contains all the initial cell classes that diversify into the multitude of neurons and glia found in older rodents. But precisely when that diversification occurs varies among brain regions: In the visual cortex, new cell types emerge weeks after birth and peak twice—once when the animal first opens its eyes and then again at the onset of the critical period, according to one study.The findings suggest that in the cortex, a cell’s fate is driven by an interaction of hardwired genetic programs and sensory experience, says study investigator Hongkui Zeng, director of the Allen Institute for Brain Science.
Outside of the cortex, however, diversification mostly takes place before birth, according to a second mouse study from Zeng’s lab. In that paper, Zeng and her colleagues charted the emergence of GABAergic interneurons—a highly diverse group of cells linked to various neurological conditions—in the mouse telencephalon.