Mice missing the autism-linked gene SHANK3 use more neurons to engage in social behavior than control mice do, reflecting a more disorganized, less efficient brain signaling network, according to a new study.
Mutations in SHANK3 show up in 1 to 2 percent of autistic people and can also lead to a related condition, Phelan-McDermid syndrome. The gene encodes a protein that supports, among other proteins, a receptor for glutamate, an excitatory chemical messenger that encourages neurons to fire. Experts suspect that an imbalance between excitatory and inhibitory signaling underlies autism traits.
In the new study, scientists monitored brain activity in freely moving mice to determine which groups of neurons, or ensembles, activate during social behavior. Mice missing a copy of SHANK3 had more neuronal ensembles that activated in concert compared with control mice, a neural network analysis shows. But the ensembles did not necessarily work together: The SHANK3 mice had more random overlap, with neuronal ensembles that are not involved in social behavior also becoming active.
The findings suggest that SHANK3 mutations disrupt the ratio of signal to noise in the social brain network, says lead researcher Vikaas Sohal, associate professor of psychiatry and behavioral sciences at the University of California, San Francisco.
“This study addresses a very important topic in neuroscience that we just don’t know much about, which is how the brain represents social information,” says Alex Kwan, associate professor of psychiatry and neuroscience at Yale University, who was not involved in the new work. “[It] provides some advance toward that understanding.”
Ensemble cast:
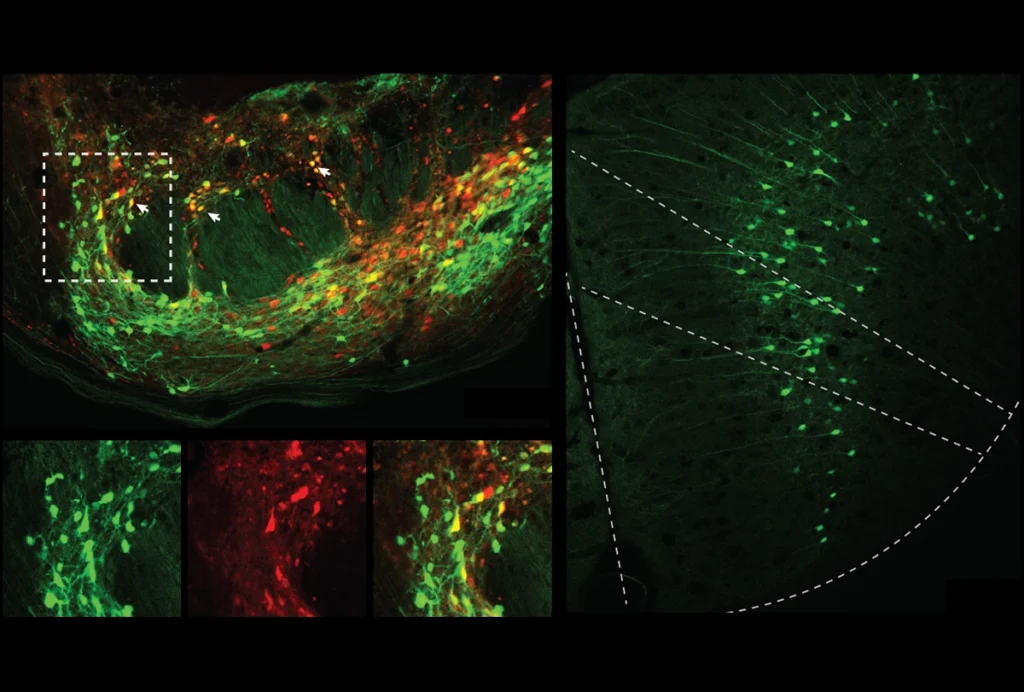
Sohal and his team recorded the activity of neuronal ensembles in mice, both in their home cage and in the presence of an unfamiliar young mouse, using miniature microscopes implanted in the animals’ prefrontal cortex. The microscopes detect the influx of calcium ions into neurons, which occurs when the cells fire.
Distinctive patterns of activity emerged in control mice: Some ensembles became more synced once a new mouse showed up, whereas others became less so.
The team fed these data into a series of algorithms to simulate the response of downstream neurons — the ones that might translate prefrontal cortex activity into behavior. And they tasked this neural network with classifying the animal’s behaviors as social or nonsocial, based solely on the activity of the neuronal ensembles.
“This allows them to isolate the ensembles of neurons that are most informative regarding the state of the mouse,” says Ofer Yizhar, professor of neuroscience at the Weizmann Institute of Science in Rehovot, Israel, who was not involved in the study. “This is a really interesting approach since it forms a sort of brain-computer ‘hybrid,’ which allows them to precisely characterize the information that the recorded neurons might be providing to the neurons ‘listening’ to them in the brain of the mouse.”
SHANK3 mice spent significantly less time interacting with unfamiliar mice than did their wildtype littermates, the team found. And their neuronal ensembles were more active yet less informative about whether the mice were in a social situation or not, the neural network showed.
The findings were published in PLOS Biology in May.
Unknown players:
The results seem to suggest that “the cortical network in these mice is less efficient in transmitting the required information,” Yizhar says. And that jibes with a 2019 study from Yizhar’s team that showed that social deficits in mice missing the autism-linked gene CNTNAP2 are accompanied by excessive noise in the prefrontal cortex.
Even so, calcium signaling recordings do not illuminate the full extent of brain activity that occurs during social interactions, Yizhar says. They show which neuronal ensembles are active and when, but they lack the sensitivity to reveal the activity of individual neurons.
And although the study combines a state-of-the-art technical approach with a novel analysis technique, it raises new questions, too, Kwan says. The imaging technique does not include the cells receiving a signal during social interactions, just the ones sending it.
“What is the nature of these cells?” Kwan says. “What is the downstream region that reads out this information and actually enacts this behavior?”
The team plans to address these questions by imaging multiple brain regions at once, says co-investigator Nicholas Frost, assistant professor of neurology at the University of Utah in Salt Lake City.
His team has also begun looking at how different neuronal ensembles are recruited during social behaviors versus anxiety-related behaviors in mice.