Protein tug-of-war controls pace of synaptic development, sets human brains apart
Human-specific duplicates of SRGAP2 prolong cortical development by manipulating SYNGAP, an autism-linked protein that slows synaptic growth.
At first glance, the mice in Pierre Vanderhaeghen’s lab in Leuven, Belgium, seem unremarkable. But inside their tiny heads, their cerebral cortex contains a mix of mouse and human neurons at two stages of development: Their native synapses are fully mature, but the connections formed from human cells are delayed and comparable to those of a newborn human baby.
Vanderhaeghen and his colleagues are studying the chimeric mice to explore this drawn-out process of synaptic development, a feature that distinguishes human brains from those of other mammals.
Many aspects of human brain development proceed slowly—neurogenesis, myelination, gliogenesis—but synaptic maturation is particularly protracted. In the prefrontal cortex, for instance, some synapses don’t fully develop until a person reaches their mid-20s.
Deviations from this maturation rate could mean that “milestones won’t be reached at the same time” and might underlie some forms of autism or intellectual disability, says Vanderhaeghen, professor of neurosciences and group leader at the VIB-KU Leuven Center for Brain and Disease Research.
An evolutionarily conserved protein called SRGAP2 controls this timing in most mammals. Humans, however, have partially duplicated copies—SRGAP2B and SRGAP2C—that inhibit the ancestral protein, two teams reported in 2012.
Like other duplicated genes found only in humans, SRGAP2 resides in a repetitive—and therefore unstable—part of the genome, says Evan Eichler, professor of genome sciences at the University of Washington, and an investigator on one of the 2012 studies. “These regions create liability by predisposing us to genomic rearrangement, [but] to persist in the population, they must have an advantage. It’s part of the cost of what it is to be human.”
SRGAP2B/C might give humans an evolutionary edge by providing more time for the environment to shape neuronal circuits, boosting our capacity for flexible learning and adaptive behavior, Vanderhaeghen and others suggest. Work from multiple groups this past year shows that the copies buy this time by promoting the synaptic accumulation of SYNGAP, an autism-linked protein.
In this regard, “[the research] has an impact beyond just understanding evolution,” says Franck Polleux, professor of neuroscience at Columbia University, who collaborates with Vanderhaeghen and worked on the other 2012 study.
V
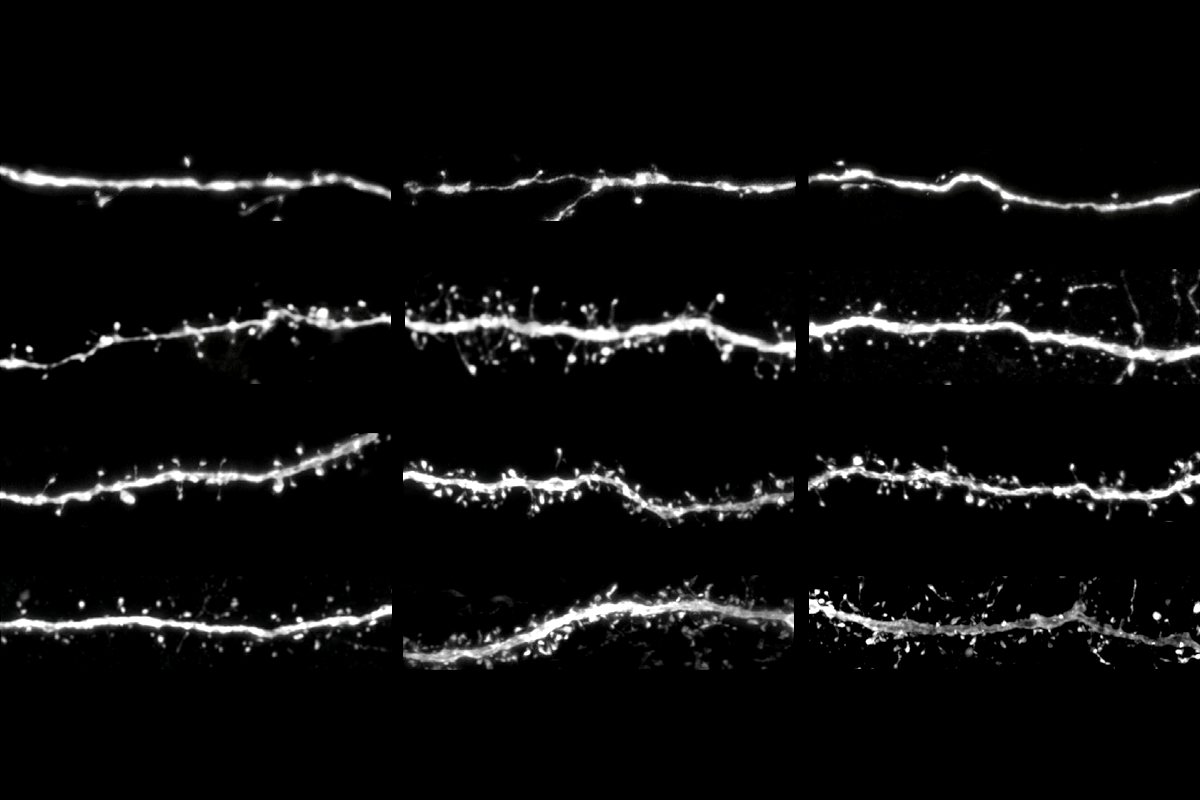
anderhaeghen’s chimeric mice have made it possible to delve deeper into how SRGAP2 and SRGAP2B/C influence synaptic development—and this process’s link to neurodevelopmental conditions. Mice engineered to express SRGAP2C form synapses that mature at slower, human-like time scales, according to Polleux’s 2012 study. The “humanized” mice also have more cortical connections and perform better than controls on tests of sensory discrimination.SYNGAP has the opposite effect on synaptic development: Mice that lack one copy of the SYNGAP1 gene, for example, show premature maturation of excitatory connections in the neocortex and a shorter window of plasticity for the environment to shape cortical circuits, a 2013 study found.
In fact, SRGAP2 and SYNGAP participate in a molecular tug-of-war that determines the rate of synaptic development, Vanderhaeghen and Polleux reported last year. Cultures of human neurons missing a single copy of SYNGAP1 show elevated synaptic levels of SRGAP2. Likewise, blunting SRGAP2 in those neurons boosts SYNGAP expression.
“It’s the first proof of principle that a pathogenic mutation associated with autism and intellectual disability leads to accelerated synapse maturation,” Vanderhaeghen says.
In most mammals, levels of both proteins are relatively balanced, maintaining synaptic maturation at a steady pace. But in people, SRGAP2B/C’s repression of SRGAP2 tips the balance in favor of SYNGAP to decelerate synapse maturation, Vanderhaeghen and Polleux say. And in people with loss-of-function variants in SYNGAP1, that equilibrium is flipped—SRGAP2 accumulates in the synapse and speeds up development, they add.
That increased pace has a direct impact on cortical circuits, Vanderhaeghen says. Cortical circuits in mice transplanted with human neurons lacking SYNGAP1 fire in response to visual stimuli three months earlier than control neurons, according to a 2024 study by Vanderhaeghen and his team. found.
“Somehow, when synapses are accelerated, functional development at the circuit level is also accelerated,” he says. That result might seem obvious, he adds, but until this study it was thought that homeostatic mechanisms might kick in to maintain the typical timing.
Making matters more complex, SRGAP2C appears to stall synapses through another autism-linked protein, CTNND2, which recruits SYNGAP to synapses, according to a 2024 Cell Reports paper. Deleting CTNND2 from transplanted human neurons accelerates synaptic development by amping up SRGAP2, just as suppressing SYNGAP1 does.
The findings underscore the fact that the speed of synaptic development isn’t controlled by a single protein but instead by multiple proteins with contrasting effects, says Cécile Charrier, research director at Institut National de la Santé et de la Recherche Médicale (INSERM) and group leader at the Institut de Biologie de l’École Normale Supérieure (IBENS), who worked on the CTNND2 study and has previously worked with Polleux to characterize the functions of SRGAP2 proteins.
B
eyond proteins, non-neuronal cells may also shape the sluggish development, or neoteny, of human synapses, Polleux says. Human microglia transplanted into rodents’ brains mature at a slower rate than the animals’ own microglia do, but only when SRGAP2B/C is expressed, according to a 2024 preprint from Polleux’s group.Likewise, mouse microglia engineered to express the human genes take longer to reach the highly branched state associated with surveilling cells. And the rodents’ synapses sport more dendritic spines than those in mice with unaltered microglia, the study also found. The human-specific genes might help to match the timing of synaptic maturation to that of microglia maturation, Polleux says.
Transplanting human neurons alongside cultured human microglia and astrocytes—something Vanderhaeghen says he is discussing with Polleux—could help to determine the extent to which synaptic neoteny is regulated by internal or external processes, Vanderhaeghen says.
In the long term, Polleux, Charrier and Vanderhaeghen say they plan to analyze other human-specific gene duplications to see if they are also implicated in the evolution of human cognition and neurodevelopmental conditions. And by assaying these genes—located in repetitive regions that are often excluded from genetic analyses—researchers may account for some unexplained cases of autism, Eichler says.
Polleux, Charrier and Vanderhaeghen say they also plan to test the hypothesis that slow synaptic development enhances cognitive functions by extending the brain’s “critical period,” and whether that window is curtailed in people with neurodevelopmental conditions.
In support of that idea, loss-of-function variants in SYNGAP1 impair learning, whereas upregulation of the protein boosts cognitive function in mice, a 2022 study found. But it’s unclear whether learning difficulties linked to SYNGAP1 variants are caused by accelerated synaptic development or by disruption in other functions, says Gavin Rumbaugh, professor of neuroscience at the Herbert Wertheim UF Scripps Institute for Biomedical Innovation & Technology, who was an investigator on the 2022 study but was not involved in any of the SRGAP2 work.
“Yeah, there’s a lack of synaptic neoteny in the [SYNGAP1] heterozygous knockout mice, but all kinds of other things happen that are probably more important for circuit development and function,” Rumbaugh says. “For example, SYNGAP has a massive effect on dendritic arborization, which has a much more significant effect on circuits.”
Most of SYNGAP’s reported functions, however, come from studies in adult mice, so it’s unclear whether other brain changes drive cognitive impairments or are simply knock-on consequences of rushed synaptic development, Vanderhaeghen says.
What’s more, phenotypic analyses of SYNGAP-deficient mice at different stages of development give rise to varied—and often conflicting—results. “Us, and many other labs, are trying to reframe phenotypic analysis by looking at the developmental trajectory of those traits, which might explain some of the conflicting evidence,” Polleux says.
Recommended reading
Astrocytes orchestrate oxytocin’s social effects in mice
Post-infection immune conflict alters fetal development in some male mice
Explore more from The Transmitter
Protein interactions important to SYNGAP1-related conditions; and more
Autism-linked copy number variants always boost autism likelihood