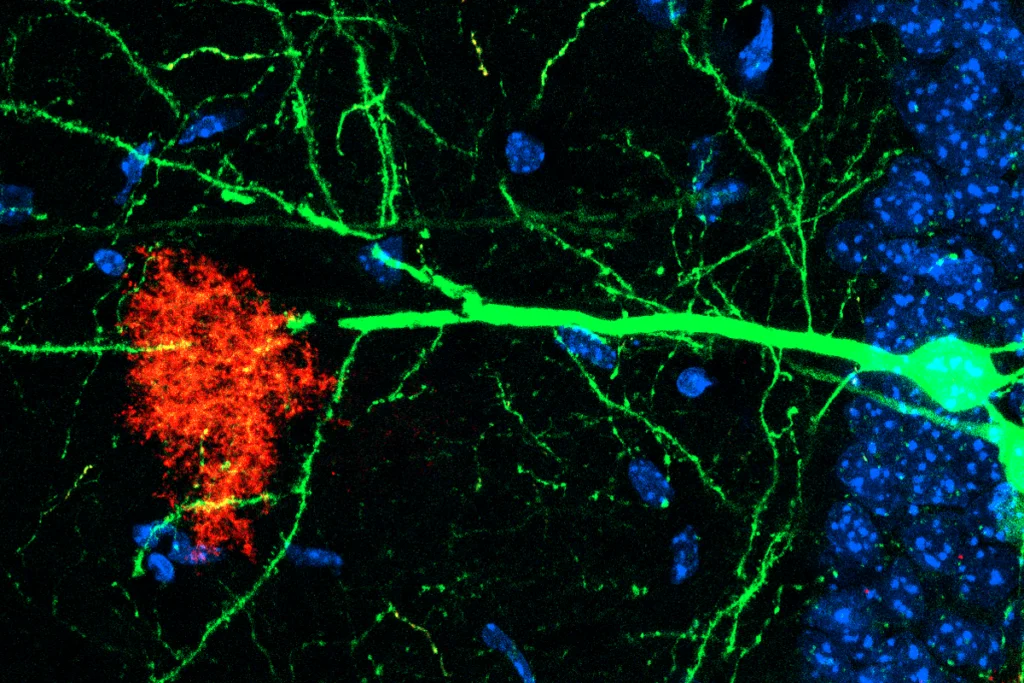
Astrocytes sense neuromodulators to orchestrate neuronal activity and shape behavior
Astrocytes serve as crucial mediators of neuromodulatory processes previously attributed to direct communication between neurons, four new studies show.
When it comes to cognition and behavior, neurons usually take center stage. They famously drive everything from thoughts to movements by way of synaptic communication, with the help of neuromodulators such as dopamine, norepinephrine and certain immune molecules that regulate neuronal activity and plasticity.
But astrocytes play essential roles in these processes behind the scenes, according to four independent studies published in the past two months. Rather than acting solely on neurons, neuromodulators also act on astrocytes to influence neuronal function and behavior—making astrocytes crucial intermediates in activities previously attributed to direct communication between neurons, the studies suggest. For instance, norepinephrine sensitizes astrocytes to neurotransmitters and prompts them to regulate circuit computations, synapse function and various behaviors across diverse animal models, three of the studies—all published last month in Science—show.
“Do neurons actually signal through astrocytes in a meaningful way during normal behavior or normal circuit function?” asks Marc Freeman, senior scientist at Oregon Health & Science University and principal investigator on one of the Science studies. These new findings “argue very strongly the answer is yes.”
Astrocytes can also detect peripheral inflammation and modify the neurons that drive a stress-induced fear behavior in mice, according to the fourth study, published in April in Nature.
Although astrocytes are no longer thought of as simply support cells, they were still “not really considered for having a real plasticity and a real important role,” says Caroline Menard, associate professor of psychiatry and neurosciences at the University of Laval, who was not involved in any of the new studies. Now “there’s more consideration from the field that behavior is not only driven by neurons, but there’s other cell types involved.”
The results suggest that astrocytes’ directorial capabilities merit further study. “There’s an entire level of regulation of the brain that we have been, for the most part, ignoring,” Freeman says. “We need to step back and think more about how astrocytes are inserting themselves into the equation.”
A
strocytes have long been known to regulate neurons by controlling the levels of extracellular neurotransmitters and mediating synaptic remodeling, among other activities. But the mechanisms by which astrocytes carry out such duties—and the extent of their influence—has been unclear. The three Science studies explored how neuromodulators influence astrocytes to control distinct behaviors in fruit flies, zebrafish and rodents.In ventral nerve cords isolated from Drosophila larvae, astrocytes did not respond to the neuromodulators dopamine, glutamate, acetylcholine or gamma-aminobutyric acid when they were added individually, Freeman and his colleagues found in their Science paper. But the astrocytes became sensitive to these molecules—indicated by an increase in intracellular calcium levels—after first being exposed to the fly homolog of norepinephrine.
The sensitivity to dopamine, which Freeman and his colleagues explored further, occurred because the cells ramped up the number of dopamine receptors on their surface, the team found.
The astrocytes’ heightened dopamine sensitivity led, in turn, to enhanced activity in dopaminergic neurons, which is critical to the larvae’s ability to “right” themselves after being placed on their side. The results show that astrocytes can sense their cell state and dynamically alter their response to neuromodulators, with important effects on behavior.
Beyond their role in the fly’s motor response, astrocytes can also mediate more complex computational decisions and state transitions, according to one of the other Science studies, which looked at larval zebrafish. In these fish, astroglia (cells similar to mammalian astrocytes) control the decision to give up, previous work suggests: When the fish swim in a virtual-reality setup that makes it seem like they are not moving forward, norepinephrine increases and signals through astroglia to turn on inhibitory neurons that suppress swimming. In this process, astroglia signal to the inhibitory neurons by releasing ATP, which then gets converted into adenosine, the new study shows.
Similarly, norepinephrine signals through astrocytes, not neurons, to influence synaptic remodeling in mouse brain slices, according to the third Science study. Exposing these slices to norepinephrine led to a decrease in synaptic strength, but not when the team used a tool called iβARK to prevent the astrocytes’ norepinephrine-induced calcium elevation or when they knocked out the norepinephrine receptors in these cells. Again, astrocytes regulated this process by releasing ATP that is converted to adenosine.
“One big takeaway for me is that, because they’re all in different species—flies, fish and mice—it really points to the fact that this signaling axis by which astrocytes respond to norepinephrine is really evolutionarily conserved,” says Misha Ahrens, senior group leader at the Howard Hughes Medical Institute’s Janelia Research Campus, who led the zebrafish work but was not involved in any of the other new studies.
A
side from these newly discovered roles regulating typical neuronal function, astrocytes actively contribute to a more pathological condition brought about by chronic stress, the new Nature study suggests.After 18 days of being restrained for six hours each day, the mice in this study froze significantly more during fear conditioning than their counterparts that had not been restrained. This type of chronic stress elicits immune responses in the periphery and promotes trafficking of monocytes into the meninges, the team found when they tracked the cells using fluorescence-activated cell sorting.
Because astrocytes can contact neurons and the meninges at the same time, it seemed possible that they link the animals’ inflammatory response to their heightened fear, says Michael Wheeler, assistant professor of neurology at Harvard University and the study’s principal investigator.
In the amygdala, which regulates fear responses, the stressed mice showed decreased expression of the gene EGFR in a subpopulation of astrocytes. This subpopulation clustered near neurons that upregulated their expression of NR2F2, suggesting that there is crosstalk between the two cell types. Getting rid of the NR2F2 in amygdala neurons decreased the animals’ stress-induced fear behavior, the study shows.
Blocking or ablating the infiltrating monocytes similarly diminished the fear behavior, and it also normalized EGFR expression in astrocytes. This result suggests that astrocytes were being “orchestrated at a distance” by immune molecules to influence behavior, says Rosemary Bagot, associate professor of behavioral neuroscience at McGill University, who was not involved with the study.
“It really adds weight to the astrocytes as drivers of the effects of stress on behavior,” says Ciaran Murphy-Royal, assistant professor of neuroscience at the University of Montreal, who was not involved with the study. “The astrocytes are really, really sensitive to stress.”
One caveat is that the team studied only male mice, which could conceal sex differences, particularly in stress paradigms, Murphy-Royal says. For example, early-life stress in mice decreases hypothalamic astrocyte activity in females but increases activity in males and ultimately leads to different firing patterns in orexin neurons, his unpublished work shows.
Anonymous commenters have pointed out on PubPeer that the cell population Wheeler’s team characterized as astrocytes might instead be neurons or contaminated with other cell types—an issue that has been brought up in other papers that similarly used RNA sequencing to identify the cells. Wheeler says the subpopulation he and his colleagues studied expressed markers consistent with astrocytes and lacked those for other cell types, and that their conclusions have been “validated across multiple independent modalities.”
U
ltimately, astrocytes could be an intermediary in many other neuronal processes, says Kevin Guttenplan, a postdoctoral fellow at Oregon Health & Science University and an investigator on Freeman’s study. “There’s no telling how many other instances there are like that in the nervous system.”For instance, astrocytes may pick up diffuse neuromodulatory signals to influence behaviors such as hunger, sleep and arousal over longer periods of time, Ahrens says. “They’re well positioned to do slower computation, such as the maintenance of these behavioral states,” he says.
And astrocytes may respond to neuromodulators differently than neurons do, Guttenplan says. In his study, norepinephrine-sensitized astrocytes that had increased calcium levels activated neurons, whereas previous studies show that they inhibit them. “There isn’t as clear a relationship between astrocyte calcium and what it does as there is in neurons,” he says. “We have to start thinking of astrocytes as their own cells with their own rules as we start to dissect these circuits.”
Now that more scientists are starting to recognize the importance of these glial cells in influencing brain activity and behavior, and have improved tools, the answers these and other open questions will emerge, Murphy-Royal says. “It’s pretty cool. We’re in the golden age of astrocytes.”
Recommended reading
Astrocytes stabilize circuits in adult mouse brain
Astrocyte networks span large swaths of brain
Unexpected astrocyte gene flips image of brain’s ‘stalwart sentinels’
Explore more from The Transmitter
Astrocytes star in memory storage, recall
Novel neurons upend ‘yin-yang’ model of hunger, satiety in brain