Neurons get most of the hype in neuroscience. But for the past few decades, researchers have been steadily building an understanding of glia, the most numerous cell type in the nervous system. Largely kicked into the spotlight by pioneer Ben Barres and others in the early 2000s, we now understand that glia serve many roles. The friendly neighborhood Spider-Man of the brain, they help to maintain the status quo by secreting growth factors and promoting the formation and function of synapses.
But in response to injury or disease, some glia can transform into cellulae non grata. Specifically, researchers have been interested in how a particular kind of glia, the astrocyte, transforms into a reactive, villainous form, not unlike when Peter Parker goes a bit dark in the movie “Spider-Man 3.”
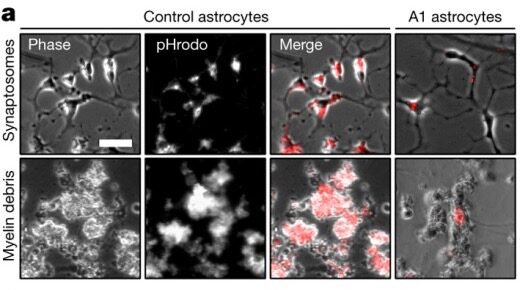
Although “reactive astrocytes” were first described as early as the 1800s, little was known about how the cells took on this dark form and what happened genetically or functionally when they did. In 2017, a group in Ben Barres’ lab at Stanford University, led by Shane Liddelow, demonstrated that microglia, another form of glia, act as the trigger. Currently cited by more than 5,000 papers, according to Clarivate’s Web of Science, Liddelow et al. (2017) comprehensively showed that these microglia secrete three cytokines that are necessary and sufficient to convert astrocytes from a protective role to a harmful one. The resulting reactive astrocytes then release neurotoxic factor(s) and, in doing so, may play a role in many different neurological diseases. As such, it’s a great paper to elucidate the fine line between helpful and hurtful in the nervous system.
Where this paper might fit
At its core, Liddelow et al. (2017) is a beautiful cellular biology paper. If you want to teach students about glial cell types, cell culture, gene expression or protein measurement, it is a great choice. Although the sequencing techniques in this paper are a bit outdated by 2025 standards—the researchers primarily used quantitative PCR (qPCR)—the use of these simpler techniques gives anyone teaching -omics a chance to talk about more foundational methods.
That said, there are many experiments packed into this paper, which you could take or leave, depending on what your students know (or don’t need to know). For example, the authors used electrophysiology to investigate the function of reactive astrocytes, but you could skip this if it is outside the scope of your course.
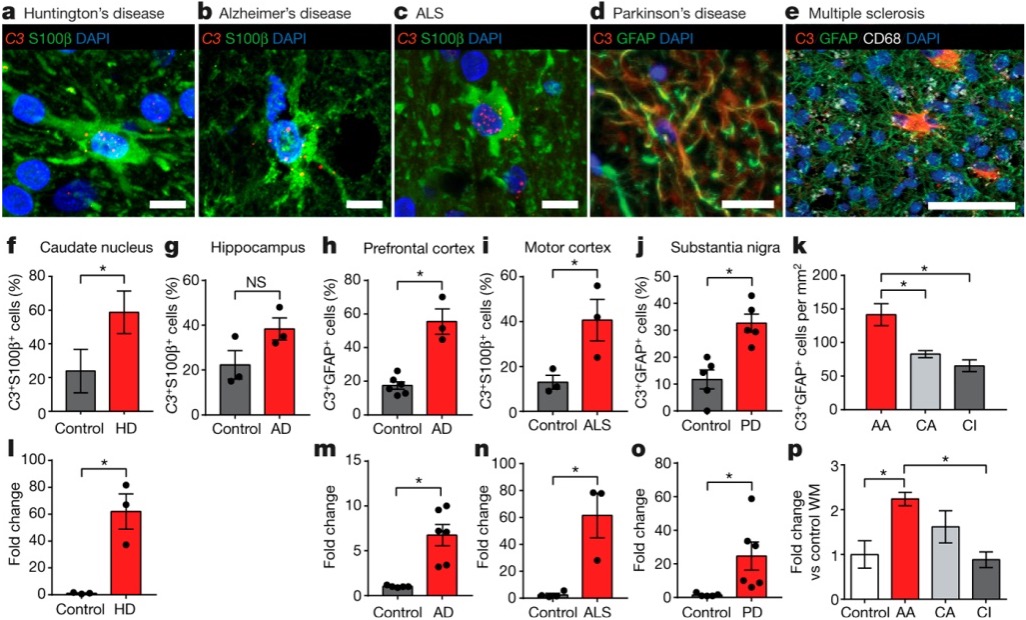
Something I love about this paper for an educational context is the immediate link to diseases that students (and the public) will care about. The inclusion of multiple disease states makes this a paper that may even be appropriate for a more clinically focused course, and it opens a path for conversations about how basic neuroscience can be translated into treatments.
Finally, you might use this paper to emphasize the importance of having the right techniques to ask the right questions. Until work that was published in 2011, it was impossible to culture astrocytes in a dish without serum, an artificial way to keep cells alive and one that likely alters astrocytes. The serum-free method, combined with insights from a different lab about the possible transcriptomic differences between astrocyte types, created the perfect foundation for Liddelow to embark on this project.
Working through the figures
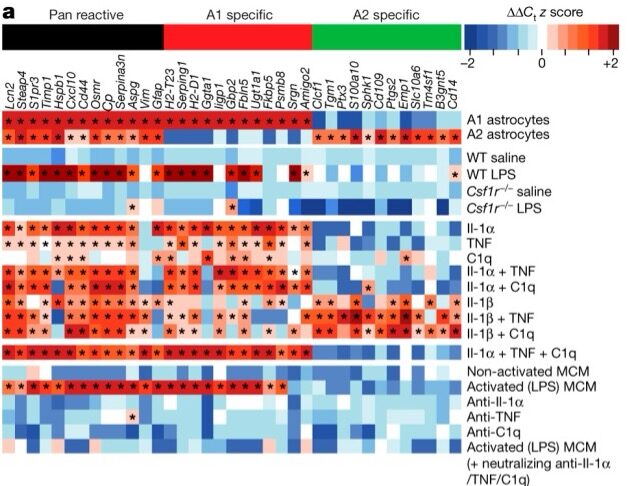
Liddelow and his colleagues began by screening many molecules that they hypothesized would induce reactivity in astrocytes. They then confirmed that a combination of three cytokines was necessary to produce reactivity and that this reactivity didn’t occur in mice without microglia (Csf1r–/-). In a series of experiments that are packed into Figure 1a, they show that both mice and cultured cells without microglia do not produce reactive astrocytes in response to an injection of lipopolysaccharide (LPS). This chemical had previously been shown to induce reactive astrocytes, but astrocytes cannot directly respond to it—they respond only in the presence of microglia.