Astrocyte networks span large swaths of brain
The networks are plastic, connect brain regions that aren’t connected by neurons and may enable long-distance communication between astrocytes, a new preprint shows.
Astrocytes depicted in textbooks huddle in small groups around synapses, supporting neighboring neurons but cut off from astrocytes elsewhere in the brain. In reality, though, the cells are linked through vast networks, according to a preprint posted on bioRxiv last month.
The field used to think about astrocyte networks as “local and really small,” says Ciaran Murphy-Royal, associate professor of neuroscience at the University of Montreal, who was not involved with the study. “This data is potentially showing that these are brain-wide networks,” which means that astrocytes might be able to communicate with each other across long distances, without the help of neurons, “which is really, really amazing.”
The astrocyte networks are plastic, too: One radiating from the barrel cortex of mice shrank after the mice had their whiskers trimmed every other day for four weeks. Some networks are confined to one hemisphere, others span both, and some link brain regions that aren’t connected by neurons.
It’s like there are two connectomes layered on top of each other—a neuronal connectome, and then an additional astrocyte connectome, says Isabella Farhy-Tselnicker, assistant professor of biology at Texas A&M University, who was not involved in the work.
A
strocyte networks were previously known to ferry cellular resources, acting “like an energy grid” to send glucose and lactate from low-activity areas to the synapses needing extra energy, Murphy-Royal says. In a mouse model of glaucoma, for example, astrocytes shuttle metabolites from the optic nerve of a healthy eye to the degenerating nerve tissue of an unhealthy one, a resource redistribution that improves visual acuity in the unhealthy eye but makes the healthy one vulnerable to neurodegeneration, according to a 2020 paper.Working on that project “got me thinking about how astrocyte connectivity links the fates of different brain regions,” says study investigator Melissa Cooper, a postdoctoral fellow in Shane Liddelow’s lab at New York University. “I had an inkling that astrocytes didn’t just connect everywhere; they were connecting very specific places.”
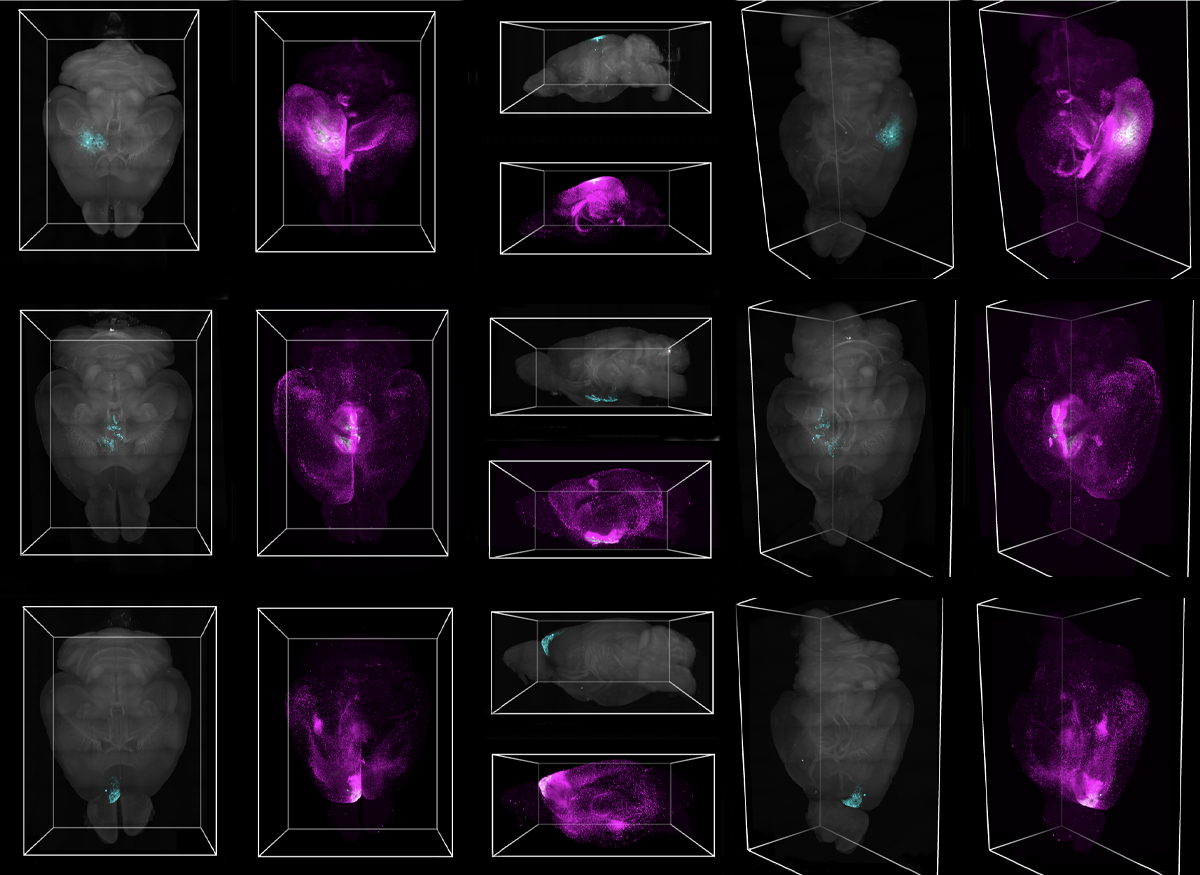
The dominant method for visualizing astrocyte networks—injecting dye into a slice of brain tissue and seeing how far it travels—limits the size of network you can observe, Murphy-Royal says: Slicing damages the cells and their connections, and even if the network is unscathed, the dye likely doesn’t have enough time to traverse the entire network, because the experiments typically take place in less than an hour.
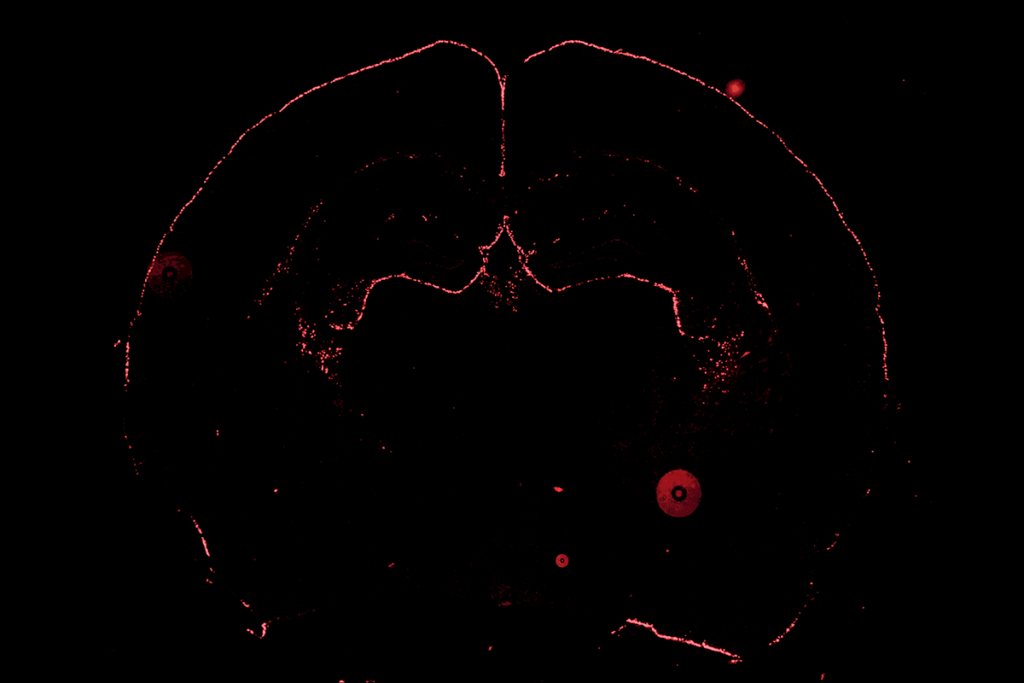
Instead, Cooper adapted a classic molecular biology technique called biotinylation that tags proteins with biotin, which is then stained with streptavidin. In her version, a viral vector fastens a biotinylating enzyme to the main protein in an astrocyte’s gap junctions, through which it communicates with other astrocytes; the enzyme, in turn, attaches biotin to everything that passes through the gap junction. The path of biotin-tagged cargo through astrocytes can then reveal the network’s size.
Cooper injected the viral vector in various brain regions of mice—the hypothalamus and the motor, barrel and prefrontal cortices—and then fed the animals biotin-laced drinking water for a week before staining and clearing the brains for imaging. Usually, Cooper says, she leaves brains to clear overnight. But the first time she ran the protocol, “I was so excited to image I just, like, waited six hours and got on the scope.”
T
he work “flips the way we think about astrocytes and what their networks may mean,” says study investigator Shane Liddelow, associate professor of neuroscience and physiology and of ophthalmology at New York University. Astrocytes play a direct role in neuronal modulation, a group of papers published earlier this year showed, and if that modulation can happen on a long-range scale, “this is a whole ’nother level of complexity for neuronal control and computation that we just haven’t thought about,” Liddelow says.The technique is “really phenomenal,” but additional control experiments would strengthen the findings, Murphy-Royal says—for example, determining if the network size changes based on how long the mice drink the biotin-laced water. It would be nice to see data demonstrating that the non-infected cells containing biotinylated molecules are truly negative for the virus, says Anna Orr, assistant professor of neuroscience at Weill Cornell Medicine, who was not involved in the research. If they were infected, the cells would produce their own biotinylated factors, which is “not indicative of connectivity to other cells.”
Follow-up work might explore which molecules move through the networks and what they do; how long-range astrocyte communication affects neuronal activity; and how the networks change during aging, neurodegenerative diseases and psychiatric disorders, Orr says.
There are plenty of other questions to explore. “If you ask 10 people” for the best thing to follow up on, Orr says, “you’d get 10 answers probably, depending on their favorite disease or process.”
Recommended reading
Astrocytes stabilize circuits in adult mouse brain
Astrocytes sense neuromodulators to orchestrate neuronal activity and shape behavior
Unexpected astrocyte gene flips image of brain’s ‘stalwart sentinels’
Explore more from The Transmitter

Astrocytes sense neuromodulators to orchestrate neuronal activity and shape behavior