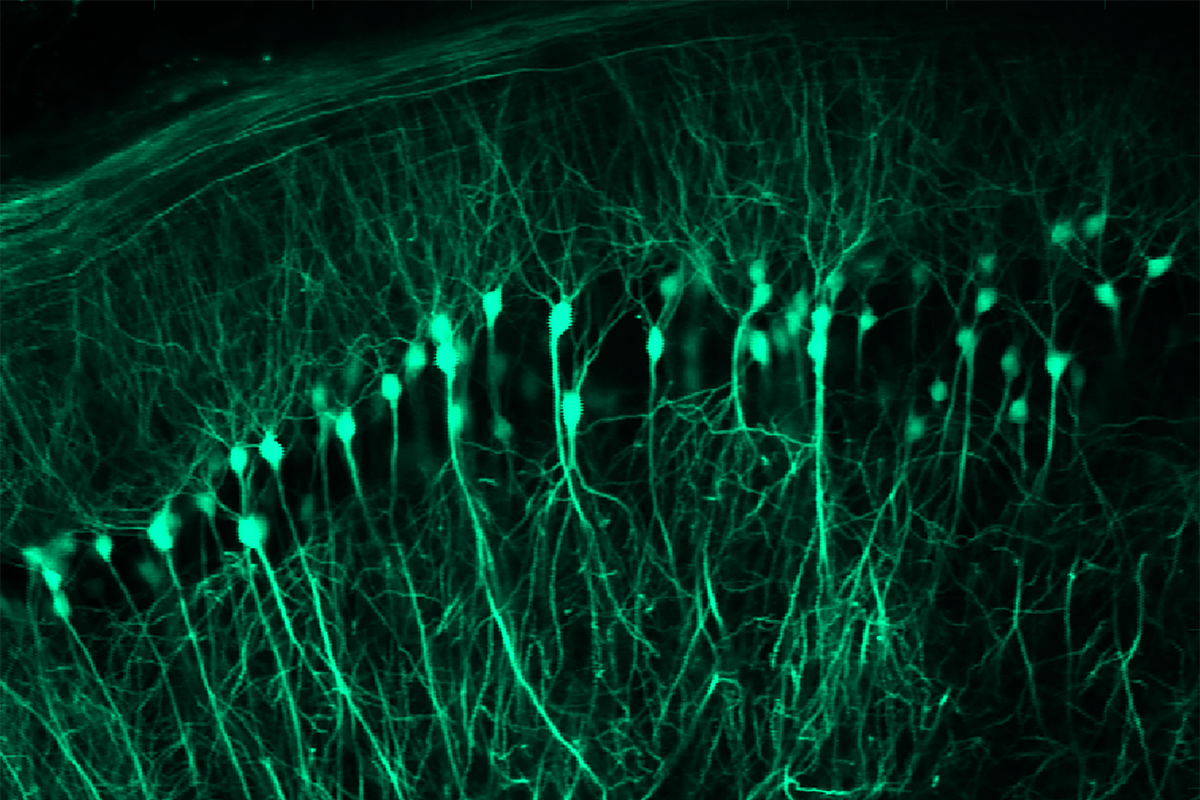
Spatial learning circuitry fluctuates in step with estrous cycle in mice
Cyclic shifts in estradiol levels coincide with changes in dendritic spine density and the activity of place cells in the CA1 region of the hippocampus, a new study shows.
The shape and density of dendritic spines fluctuate in step with the estrous cycle in the hippocampus of living mice, a new study shows. And these structural changes coincide with shifts in the stability of place fields encoded by place cells.
“You can literally see these oscillations in hippocampal spines, and they keep time with the endocrine rhythms being produced by the ovaries,” says study investigator Emily Jacobs, associate professor of psychological and brain sciences at the University of California, Santa Barbara. She and her colleagues used calcium imaging and surgically implanted microperiscopes to view the dynamics of the dendritic spines in real time.
The findings, published in Neuron in May, replicate and expand upon a series of cross-sectional studies of rat brain tissue in the early 1990s that documented sex hormone receptors in the hippocampus and showed that changes in estradiol levels across the estrous cycle track with differences in dendritic spine density.
“The field of neuroendocrinology was really changed in the early ’90s because of this discovery,” Jacobs says.
The new work is a “very important advancement,” says John Morrison, professor of neurology at the University of California, Davis, who was not involved in the research. It shows that spines change across the natural cycle of living mice, supporting estradiol’s role in this process, and it links these changes to electrophysiological differences, he says.
“The most surprising part of this study is that everything seems to follow each other. Usually biology doesn’t cooperate like this,” Morrison says.
B
efore the early 1990s, estrogens were viewed only as reproductive hormones, and their effects in the brain were thought to be limited to the hypothalamus, says Catherine Woolley, professor of neurobiology at Northwestern University, who worked on the classic rat hippocampus studies when she was a graduate student in the lab of the late Bruce McEwen. For that reason, her rat hippocampus results were initially met with “resistance,” she adds. A leader in the field once told her to “get some better advice” from her adviser “because estrogens are reproductive hormones, and they don’t have effects in the hippocampus,” she recalls.Her work also suggested that connections between neurons in the hippocampus are flexible—which was met with even more disbelief, Woolley says.
“Those papers really knocked my socks off,” Morrison says. “They went against everything that I’d been taught—that neurons and spines are stable.”
Estrogen’s effect on dendritic spine density lessens with age, according to subsequent studies in rats and monkeys. And fluctuations in estradiol and progesterone levels across the menstrual cycle or during pregnancy also correlate with structural and functional reorganization in the human brain, imaging studies suggest.
In the new work, spine density increased by 11.5 percent during proestrus, when estradiol levels are highest, and decreased by 12.4 percent during estrus, when estradiol levels drop and ovulation occurs. About 36 percent of new spines added during proestrus stuck around and were not pruned in response to fluctuations in estradiol, the work also shows. These results were consistent across two consecutive cycles in the animals.
Back-propagating action potentials—electrical signals that travel from the axon back into the dendrites to regulate synaptic strength—also increased during proestrus, the team found. The mice also showed some estrous-cycle-related changes in the activity of place cells—which typically fire whenever a mouse encounters a specific location, to help it form a mental map of its environment, but can remap the space and shift the location in which they fire if some of the visual cues or other factors change. The place cells were most stable during proestrus—they continued to fire at a particular location even if they had temporarily remapped to another location in response to a short-term environmental change—and least stable during estrus, the study shows.
T
he work suggests that hormone shifts affect not only dendrite morphology in the hippocampus but also synaptic circuitry and spatial tuning, says lead study investigator Nora Wolcott, a graduate student in principal investigator Michael Goard’s lab at the University of California, Santa Barbara. The team is working to understand if these changes affect learning and memory, she adds.And there are still many unanswered questions about the role of sex hormones across the brain—for example, how they affect other brain regions besides the hippocampus, such as the somatosensory cortex, Wolcott says. Another important area of research is understanding variations across the lifespan and in periods such as menopause, Jacobs adds.
But resistance to studying females because of misconceptions about hormonal responses persists, Woolley says. She encourages fellow researchers to view changes regulated by hormonal fluctuations as “stable” or “flexible” rather than better or worse and notes that males have hormonal modulations, too.
“If you’re studying a whole animal, you’re studying hormones, whether you admit it or you don’t,” Woolley says.
Jacobs agrees and says she hopes to see continued growth in the field of neuroendocrinology. “I think there’s hopefully going to be a revolution in neuroscience to really appreciate these steroid hormones as potent neuromodulators of learning and memory and all sorts of behaviors.”
Recommended reading

Two primate centers drop ‘primate’ from their name
Post-infection immune conflict alters fetal development in some male mice
Explore more from The Transmitter
Novel neurons upend ‘yin-yang’ model of hunger, satiety in brain