A gene therapy that compensates for a missing copy of SYNGAP1, a gene strongly associated with autism, diminishes hyperactivity and brain activity linked to epilepsy, according to a new study in mice. The therapy also normalizes brain-wave patterns linked to learning, memory, attention and sensory processing.
“This is the first published study to use a virus to supplement SYNGAP1,” says Gavin Rumbaugh, professor of neuroscience at the Herbert Wertheim UF Scripps Institute for Biomedical Innovation & Technology, who did not take part in this study. Though the treatment did not completely prevent seizures, “it’s a promising start.”
SYNGAP1 is “one of the top three genes that have been linked with autism,” says study investigator Meagan Quinlan, a scientist at the Allen Institute for Brain Science. An estimated 1 million people globally carry mutations in SYNGAP1 that can lead to autism, intellectual disability, epilepsy, motor impairment and increased risk-taking behavior.
SYNGAP1-related disorders are primarily caused by gene variants that result in SYNGAP1 haploinsufficiency, meaning that a person has just one functional copy of the gene instead of the usual two. Existing medications treat certain traits of SYNGAP1-related disorders but do not address their underlying cause.
One promising experimental therapy for the condition uses small fragments of RNA called antisense oligonucleotides (ASOs) to bind to SYNGAP1’s mRNAs to boost its expression. Although research into ASOs for SYNGAP1 has yielded compelling data, ASOs require repeated dosing at regular intervals, whereas gene therapies that rewrite a person’s genome could provide long-term benefits from a single dose, says study senior investigator Boaz Levi, an associate investigator at the Allen Institute.
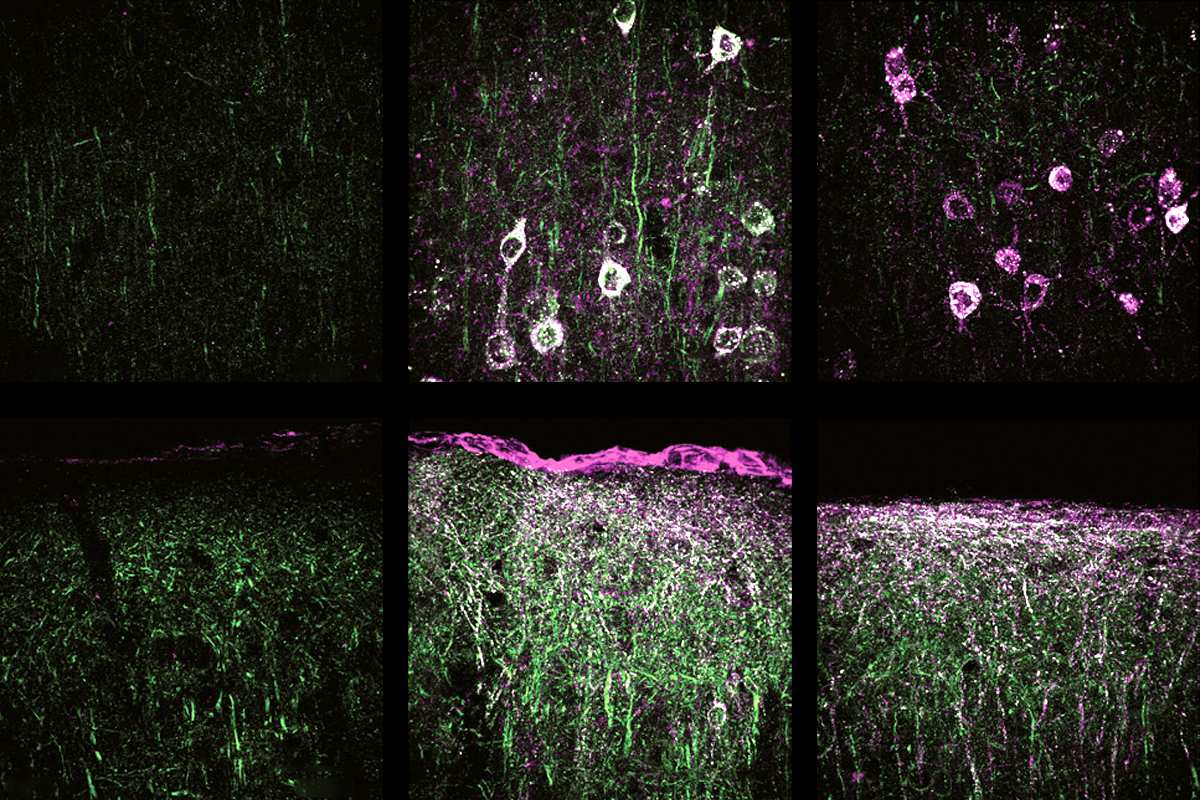
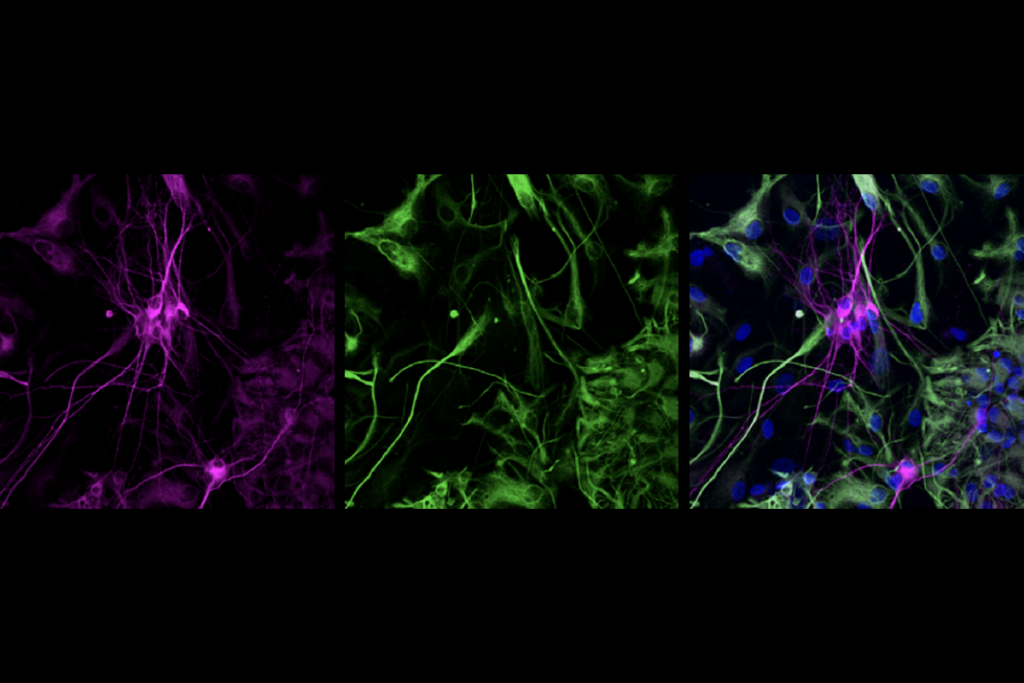
The new study delivered a working copy of SYNGAP1 through an adeno-associated virus vector that can be administered into the blood “but then crosses into the brain to efficiently target most neurons,” says study investigator Rong Guo, a scientist at the Allen Institute.
The researchers tested 21-day-old SYNGAP1 haploinsufficient mice. This developmentally corresponds roughly to ages 1 to 3 years in children, when most people with SYNGAP1-related disorders are diagnosed, Quinlan says. “Although early intervention is often considered necessary for treating neurodevelopmental disorders, our findings are particularly exciting because it suggests that some of the disease symptoms can be reversed after onset.”
S
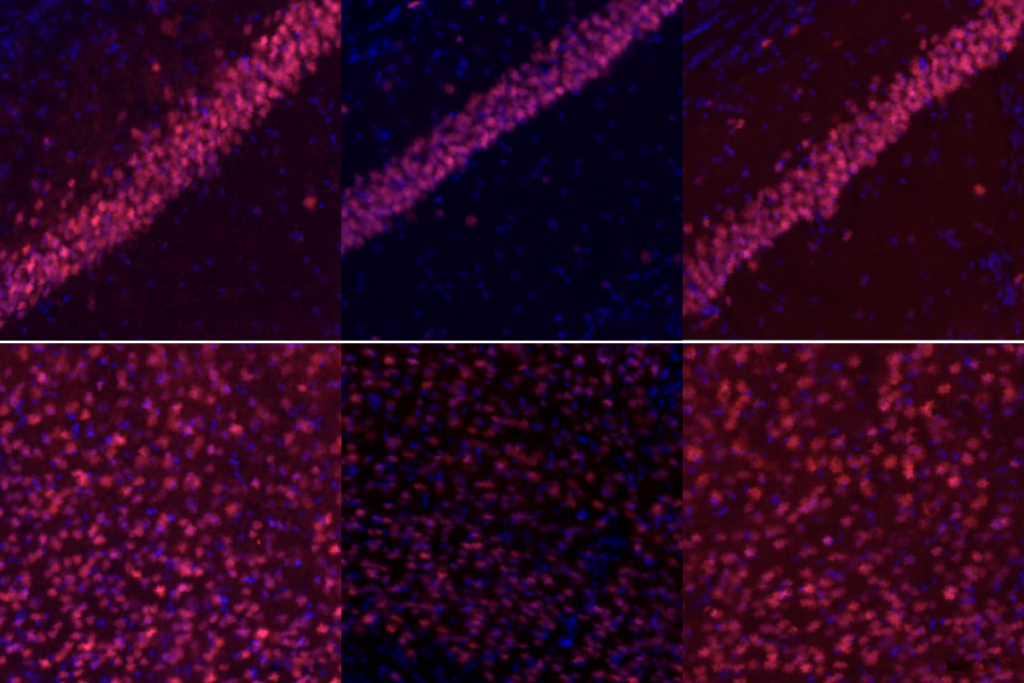
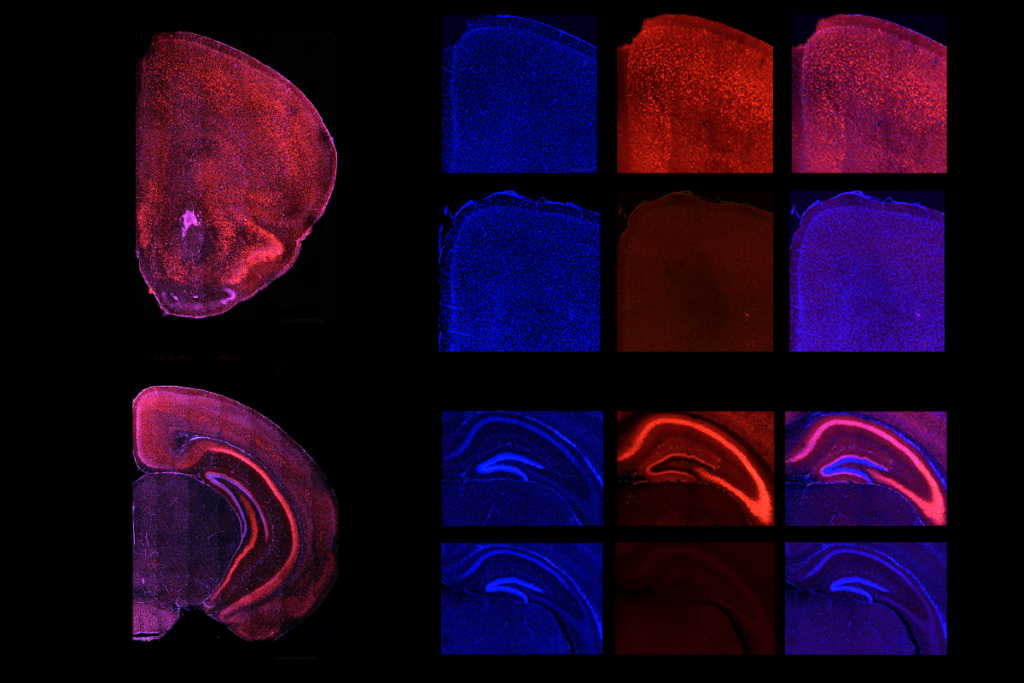
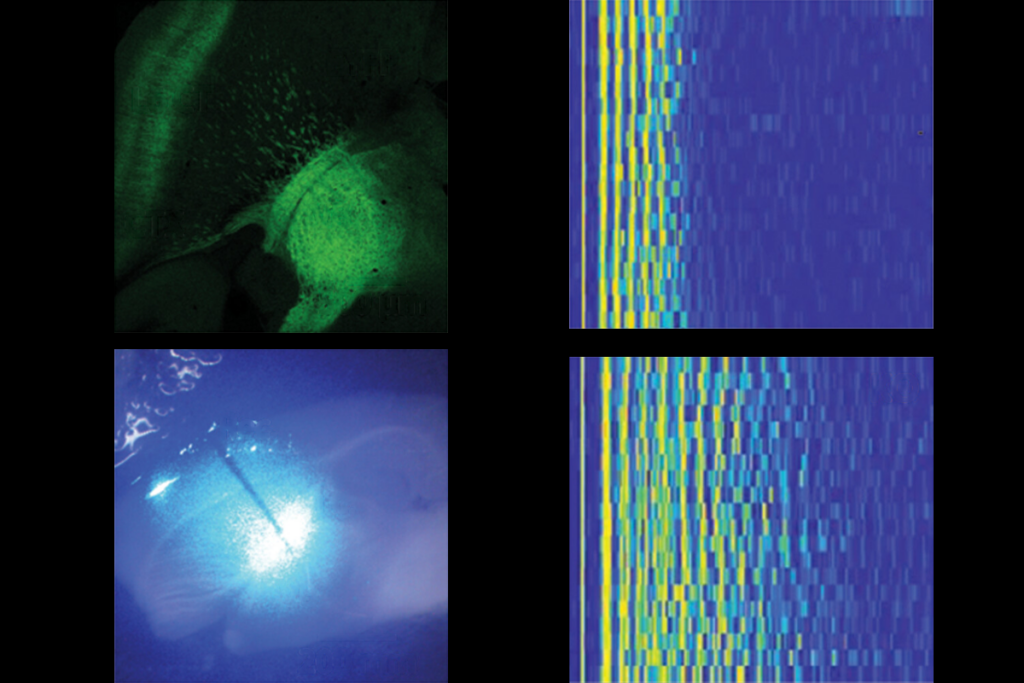
YNGAP1 encodes several SYNGAP protein isoforms, the functions of which are not fully understood. Quinlan and Guo’s team focused on the alpha1 C-terminal isoform, which helps neurons communicate at synapses and contributes to the traits of SYNGAP1 haploinsufficiency in mice, previous findings suggest.A single dose of the treatment almost completely restored SYNGAP protein levels throughout the brain in 21-day-old mice, the team found. It also decreased the number of interictal spikes, a form of brain activity linked to epilepsy, by roughly threefold. Interictal spikes can disrupt neuronal activity and are linked to cognitive impairment, so lessening these spikes might offer cognitive benefits, the investigators write in the article.
The treatment did not eliminate seizures, however. Three of the nine treated mice experienced one or more seizures. (The small number of rodents examined prevented the researchers from gauging the impact of treatment on seizure frequency, the scientists wrote.)
In addition, the treatment normalized the animals’ electroencephalogram brain-wave patterns and their performance on tests measuring hyperactivity and risk-taking behavior. Abnormal brain rhythms in SYNGAP1-related disorders are associated with cognitive issues that affect learning, memory, attention and sensory processing.
“We were surprised and impressed that the single isoform could reduce several key disease-relevant phenotypes,” Quinlan says. The scientists detailed their findings in September in Molecular Therapy.
As seizures persisted, all in all, “what we are seeing here is less-than-perfect rescue, but the good news is, even if things are less than perfect, one can still ameliorate many major neurodevelopmental phenotypes,” says Kevin Bender, professor of neurology at the University of California, San Francisco, who did not take part in this research.
Rumbaugh points out that the tag the researchers encoded into their treatment to help image the resulting molecule was located near the protein’s C terminus, which possesses key structures linked to its activity. “That may have disrupted its function,” he says. “They may have seen better results had they not placed the tag there.”
Subsequent research will have to see how effective their treatment might prove with the removal of the tag, Levi says. He notes that the fact that they saw results even with the tag means that those C terminus structures may not have been disabled by the tag, or that those structures aren’t required for the protein’s function. In the future, Levi says, he would also like to evaluate the therapeutic treatment in relation to other isoforms, greater cell-type specificity and multiple behaviors, including ones associated with autism.
He cautions that “our work cannot yet answer if one isoform is sufficient to rescue all major SYNGAP-related disorder phenotypes in the human brain.” Rumbaugh notes that future research should test other SYNGAP isoforms, alone and in combination with one another.
Levi adds that ASOs can increase expression of multiple SYNGAP1 isoforms. He suggests that ASOs and viral gene therapies for SYNGAP1 haploinsufficiency “could be applied concurrently, as neither is expected to interfere with the other.”