Tuberous sclerosis gene loss triggers autism-like features
Losing one or both copies of TSC1, one of the two genes responsible for tuberous sclerosis complex, in specific cells of the cerebellum can trigger several autism-like behaviors in mice, according to research published 1 July in Nature.
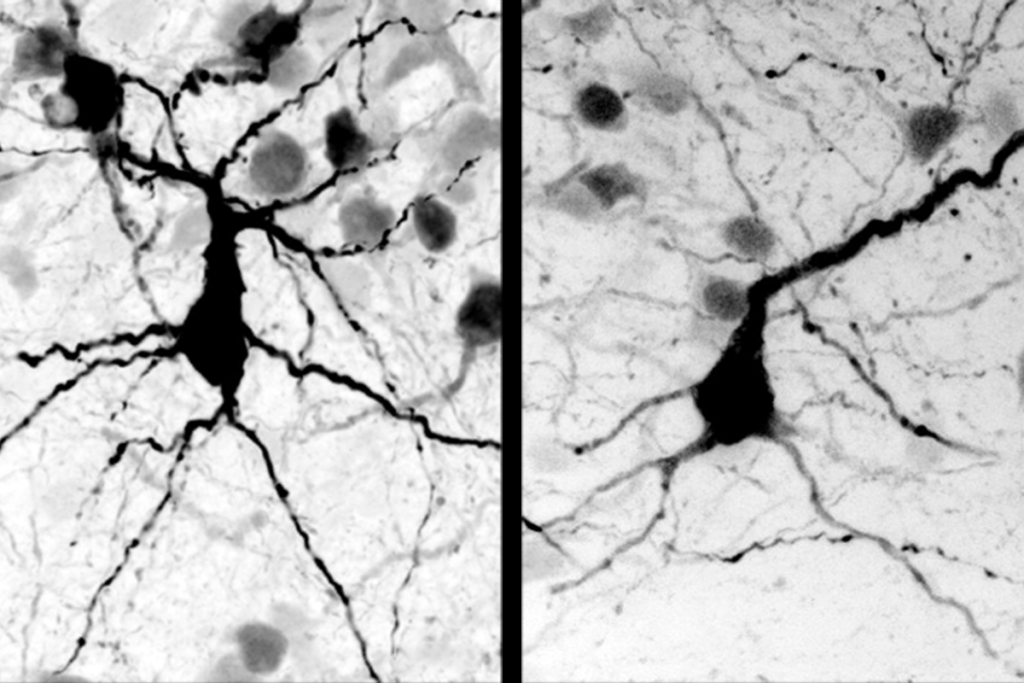
- Faulty conduit: Mutant mice missing both copies of TSC1 (right), a gene linked to tuberous sclerosis complex, have fewer Purkinje cells (red) than do controls (left) or mice with one copy (middle).
Losing one or both copies of the TSC1 gene solely in specific cells of the cerebellum can trigger several autism-like behaviors in mice, according to research published 1 July in Nature1. Mutations ineither TSC1 or TSC2 are known to cause the autism-related disorder tuberous sclerosis complex (TSC).
TSC is a rare genetic disorder characterized by benign tumors in multiple organs, including the brain and kidneys, throughout the body. Children with the disorder often develop neurodevelopmental problems such as learning disabilities, and about 50 percent are diagnosed with an autism spectrum disorder.
Mice engineered to lack one or both copies of TSC1 in Purkinje cells of the cerebellum produce more cries than control mice, have social deficits and display repetitive behaviors, all of which resemble core symptoms of autism.
Researchers were able to prevent and reverse these symptoms by treating the mutant mice with an immunosuppressant drug called rapamycin.
“If you just give [the mice] a single drug on postnatal day 7, all these developmental problems are blocked,” says lead investigator Mustafa Sahin, associate professor of neurology at Boston Children’s Hospital. “They’re essentially the same as mice that don’t have these mutations.”
Research into TSC has shown that children who have a misshapen cerebellum, a brain area important for motor and sensory coordination, are more likely to also have autism.
This high prevalence of autism, coupled with the involvement of just two genes, makes TSC an attractive model to study the cerebellum’s role in autism, the researchers say.
Purkinje cells, large branching neurons that are found solely in the cerebellum, are a good place to start because they are the only conduits for signals between the cerebellum and the rest of the brain. The mutant mice have fewer Purkinje cells than do controls, and those cells are larger than in controls, the researchers found.
Cerebellar circuits:
Dysfunction of the cerebellum has long been associated with autism, with small studies suggesting changes to its structure and the presence of fewer cells in people with the disorder.
But the classic function ascribed to the cerebellum, motor coordination, is a relatively little studied aspect of autism.
By altering a cell type found only in one part of the brain, researchers can trace the resulting effects back to a specific part of the brain, says Gene Blatt, professor of anatomy and neurobiology at Boston University School of Medicine, who was not involved in the work. “[This study] gives us an opportunity to explore further what the exact role of the cerebellum is in autism.”
The researchers say they expected that altering the TSC1 gene would disrupt motor coordination in mice. The other autism-like features they found were a surprise, says Sahin.
Loss of TSC1 is known to boost the activity of the mammalian target of rapamycin (mTOR) signaling pathway, which regulates cell growth and proliferation. This may in turn account for the tuber-shaped tumors in the brain and other organs of people with TSC.
In the new study, the researchers tracked the number of Purkinje cells in the mutant mice throughout development. In mice missing both copies of the gene, the cells decrease in number at 2 months of age and then again at 4 months, the researchers found. Those Purkinje cells that are present are smaller than normal and fire less often than those in controls.
Mice lacking only one copy of TSC1 have Purkinje cells that are normal in both number and size. But these cells, too, fire less frequently than Purkinje cells in control mice.
This means there’s less output from the Purkinje cells to the rest of the brain, says Samuel Wang, associate professor of molecular biology and neuroscience at Princeton University in New Jersey, who wasn’t involved in the study.
Both sets of mutant mice also have behavior problems. Compared with controls, they spend less time interacting with an unfamiliar animal, vocalize more at postnatal days 5 to 12, and show no preference for another mouse over an object.
“It raises the possibility that decreased cerebellar output helps shape brain circuitry in these mice as they develop autism-like behaviors,” Wang says.
Mice that lack one or both copies of the gene also have trouble adapting to changes in their environment. When required to find an escape platform in a pool, the mutant mice find their way just as quickly as control mice do. But when researchers change the platform’s location, they take longer to find the new site than control mice do.
Mice lacking one copy of the gene have no motor problems, but those missing both copies develop a stumbling, wobbly gait by 7 to 8 weeks of age.
“[These findings] give us a toehold in the circuitry that underlies autism,” says Sahin. “Essentially the whole cerebellum output is abnormal in these mice.”
Clinical trials of rapamycin to treat TSC are already under way. Sahin and his group are trying to determine whether there’s a sensitive period during development in which rapamycin treatment would be most effective.
References:
1: Tsai P.T. et al. Nature. Epub ahead of print (2012) PubMed
Recommended reading
New organoid atlas unveils four neurodevelopmental signatures
Explore more from The Transmitter
Snoozing dragons stir up ancient evidence of sleep’s dual nature

The Transmitter’s most-read neuroscience book excerpts of 2025