In 2012, neuroscientists Sebastian Seung and J. Anthony Movshon squared off at a Columbia University event over the usefulness of connectomes—maps of every connection between every cell in the brain of a living organism.
Such a map, Seung argued, could crack open the brain’s computations and provide insight into processes such as sensory perception and memory. But Movshon, professor of neural science and psychology at New York University, countered that the relationship between structure and function was not so straightforward—that even if you knew how all of a brain’s neurons connect to one another, you still wouldn’t understand how the organ turns electrical signals into cognition and behavior.
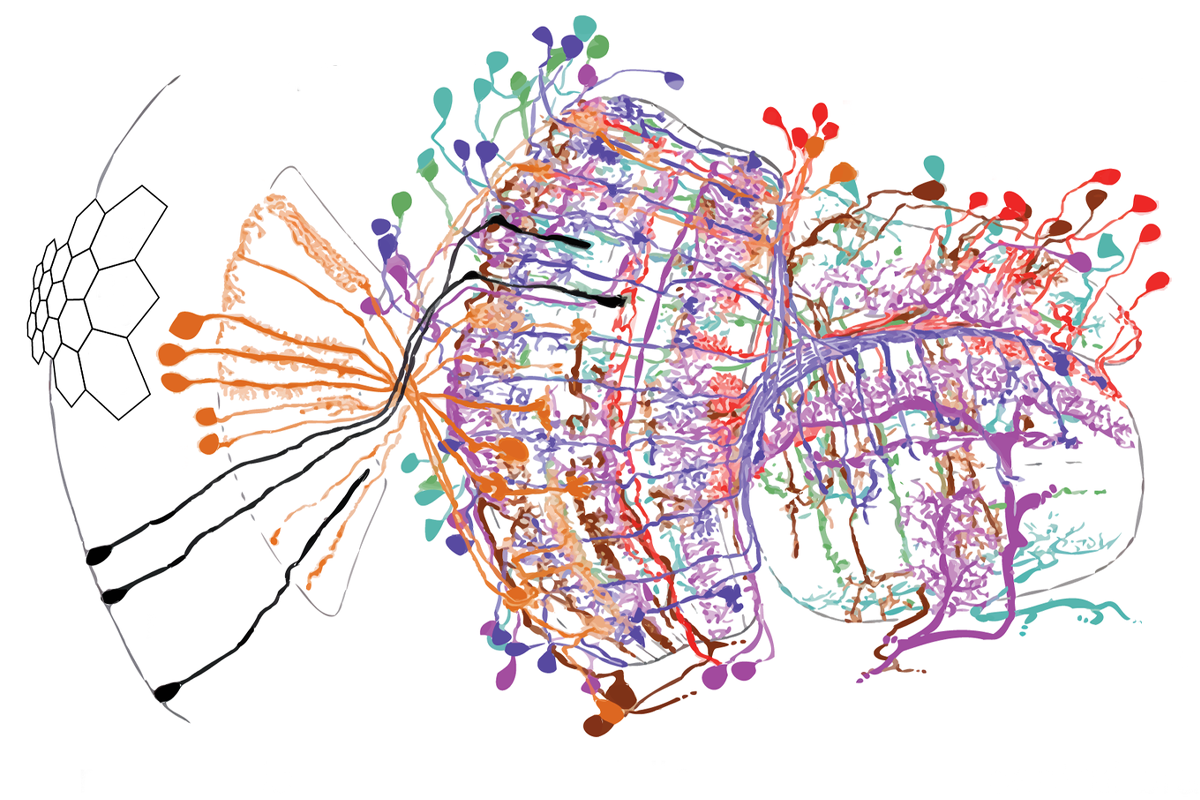
The debate in the field continues, even though Seung and his colleagues in the FlyWire Consortium completed the first connectome of a female Drosophila melanogaster in 2023, and even though a slew of new computational models built from that and other connectomes hint that structure does, in fact, reveal something about function.
“This is just the beginning, and that’s what’s exciting,” says Seung, professor of neuroscience at the Princeton Neuroscience Institute. “These papers are kicking off a beginning to an entirely new field, which is connectome-based brain simulation.” A simulated fruit fly optic lobe, detailed in a September 2024 Nature paper, for example, accurately predicts which neurons in living fruit flies respond to different visual stimuli.
“All the work that’s been done in the past year or two feels like the beginning of something new,” says John Tuthill, associate professor of neuroscience at the University of Washington. Tuthill was not involved in the optic lobe study but used a similar approach to identify a circuit that seems to control walking in flies. Most published models so far have made predictions about simple functions that were already understood from recordings of neural activity, Tuthill adds. But “you can see how this will build up to something that is eventually very insightful.”
And having the connectome has shaved years off the time it takes to, say, identify a neuron involved in a particular behavior, Seung says, and narrowed the field of experiments to only those that align with the way the brain is actually connected. “You don’t have to spend months or years chasing down dead ends,” he adds. “Simulation is going to improve that even more.”
T
But the excitement about that possibility was almost immediately matched by reservations about the time and money involved—and concerns that the payoff might not be worth it.
Some of those concerns were laid out in a seminal 2013 Nature Methods commentary by neuroscientists Eve Marder and Cori Bargmann. They wondered at the time what additional information beyond the brain’s synaptic connections—whether those connections are inhibitory or excitatory, for instance—would be needed to make truly informative models.
More than a decade later, fly connectome data still lack that basic information. They also don’t account for electrical synapses—connections between neurons via electrical signals shared across a cell membrane. And some cells can be connected both electrically and chemically, creating multiple potential pathways across a single circuit, Marder says. “In the absence of knowing who’s electrically coupled to who, you can make some assumptions from a chemical circuit connectome that are going to be missing a lot of parallel pathways.”
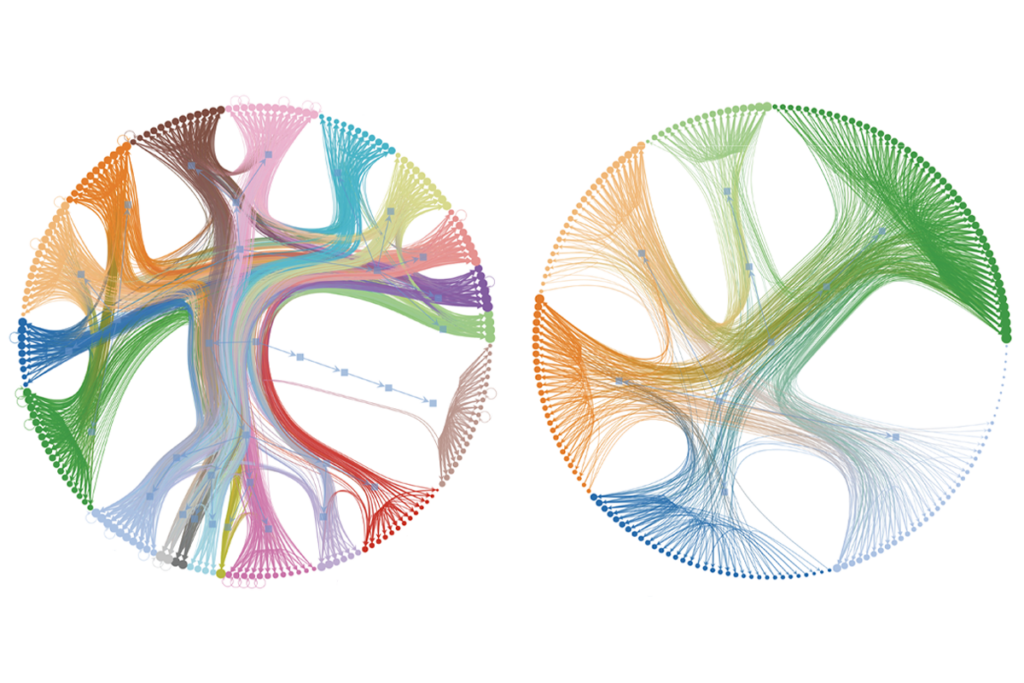
In its most pared-down form, a connectome represents the connections between neurons mathematically. In the case of the fruit fly, each connection appears in a matrix of 139,000 or so rows and columns, each representing one of the fly’s 139,000 neurons. The cells display numbers that indicate how strongly two neurons are connected. Most contain a 0, because most pairs of neurons do not touch.
Simulations based on such matrices must add information—or make assumptions—about types of neurons, where they are located in the brain and what kinds of signals they propagate. That often works: Folding neural-network predictions about neurotransmitter identities into a connectome-based simulation for taste in flies, for example, generated activity in neurons known to help move the proboscis in response to sugar. Silencing those neurons in living flies blocked the behavior, suggesting the model had found the correct cells.
But Srinivas Turaga, a group leader at the Howard Hughes Medical Institute’s Janelia Research Campus, is developing a method to incorporate more real-world data into the models’ assumptions. In the September study that modeled the fly’s visual system, Turaga and his team built some basic assumptions about visual input into 50 models—all based on a fruit fly connectome from Janelia—and then they gave the models a rule: Whatever else they do, they must have the keen vision of a fly.
As these models processed simple movies, they spat out the activity from the neurons in the optic lobe’s motion pathway, and—across all 50—that activity aligned almost entirely with data recorded in 26 studies of living flies. For example, the models correctly identified a set of neurons called elementary motion detectors, known as T4 and T5 neurons.
Neurons outside of the T4 and T5 group also seemed to compute motion, suggesting other as-yet-unknown visual-system cells exist. “Whether in reality they do this or not, that’s an empirical question,” says Turaga’s co-investigator Jakob Macke, professor of machine learning in science at Tübingen University. “For me, what this development can initiate is a new time in which computation modeling is so accurate that we can use it as a way to drive and plan experiments, rather than to explain experiments after the fact.”
In April of this year, Turaga’s team published in Nature a whole-body simulation of a fly that uses machine learning to walk and fly. That model is currently constrained by an artificial neural network, but it could instead be guided by the connectome—as well as a map of the nerve cord that connects the fly’s brain and body—in a similar way to Turaga’s optic lobe simulation.
“Just dreaming of that would have been impossible before the connectome,” Turaga says. “Even now it sounds a little crazy. I’m not embarrassed to say that. The methods we’ve developed are promising enough to say, ‘Maybe we can dream that way, and maybe we can start thinking about building those models.’”
E
For one, many of the models assume that large neurons have a proportionally large visual field—a correlation that did not appear in the flies. What’s more, pairs of cell types that shared some aspects of connectivity, such as a connection with the same input cell type, did not necessarily have similar visual responses.