Engrams in amygdala lean on astrocytes to solidify memories
Disrupting the astrocyte-neuronal dynamic in mice destabilizes their memory of fear conditioning.
Astrocytes may reinforce an animal’s recollections a day or more after an original memory forms, according to a study published today in Nature.
As soon as the animal recalls the memory, astrocytes in the amygdala activate, the work shows. Disrupting this activation quells anxiety-like behaviors in fear-conditioned mice.
“Neurons are encoding information or immediately responding, and astrocytes contribute to the processing of that kind of information,” says study investigator Jun Nagai, team leader of the Laboratory for Glia-Neuron Circuit Dynamics at the RIKEN Center for Brain Science.
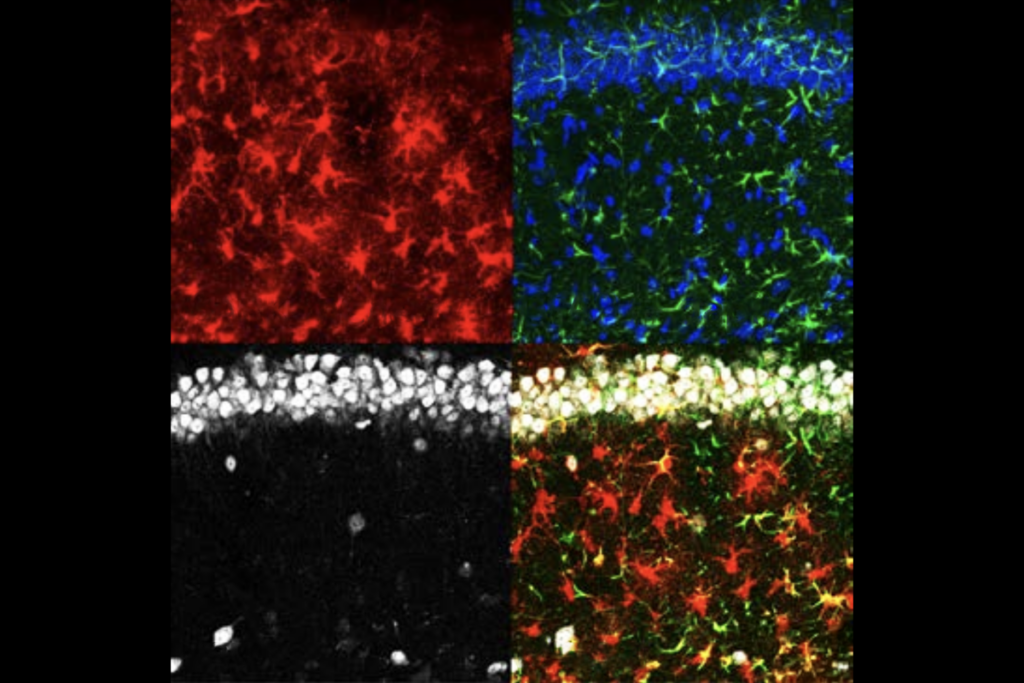
The expression of FOS, a gene often used to study experience-dependent activation in neurons, flagged which astrocytes interact with individual memory circuits, called engrams. In genetically engineered mice, a tamoxifen injection caused any FOS-expressing astrocytes to produce a fluorescent protein, which rendered the cells visible during whole-brain imaging.
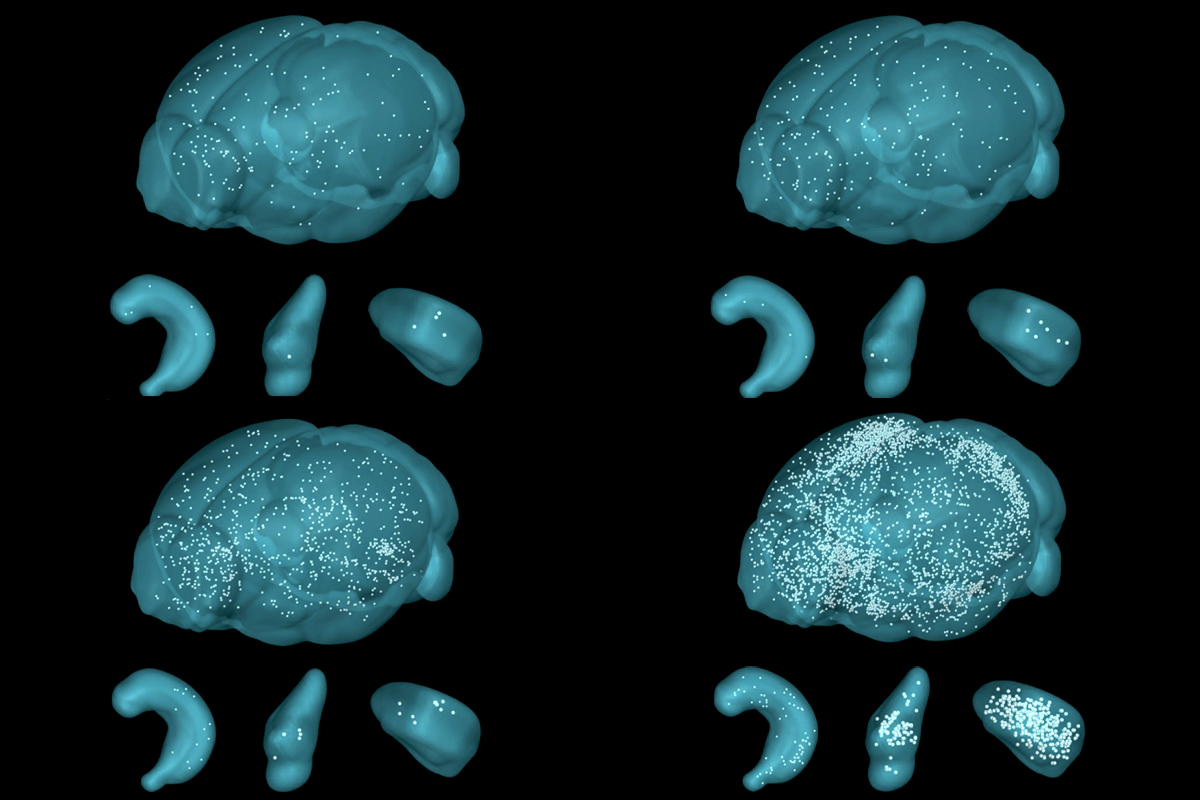
A tamoxifen injection immediately after fear conditioning, by way of a mild foot shock in a specific cage, did not reveal major increases in astrocytic FOS expression anywhere in the brain—a finding that Nagai says the team originally took as a sign that the experiment had failed.
A postdoctoral researcher examined the brains of mice that received the tamoxifen injection a day later, when the animals were placed back in the fear-conditioning cage. This time, the brains showed widespread astrocytic FOS expression, suggesting that the animal’s recall of the previous day’s shock had activated the cells. The postdoc celebrated his find with “a nice cocktail” later that Friday night, Nagai recalls.
“If I would have caught [the astrocytic FOS], I would be excited,” says Amit Agarwal, research group leader at the Institute for Anatomy and Cell Biology at Heidelberg University, who studies neuronal-glial interactions but was not involved with new study. Astrocytic FOS expression has proven elusive in his and others’ experiments, he says.
Agarwal credits Nagai and his team’s success in part to their whole-brain imaging method, as they may not have captured the relatively small population of FOS-expressing astrocytes had they instead relied on immunostained brain slices, he says.
T
he astrocytic response to fear recall was most pronounced in the amygdala, Nagai’s team discovered. This activation relied on two inputs: glutamate from surrounding neurons and noradrenergic projections from the locus coeruleus. The results are consistent with a 2014 study by Agarwal and his colleagues that described how noradrenaline primes astrocytes to respond to local neuronal signals. More recently, three separate papers published in Science in May elaborated on noradrenaline’s influence on astrocyte-neuron dynamics.Astrocytes expressed more noradrenaline receptors after fear conditioning, Nagai’s new work shows. The density of these G protein-coupled receptors peaked one day after conditioning, around the same time that the researchers observed peak FOS expression during fear recall. Knocking down the noradrenergic receptors depressed recall-associated FOS expression in astrocytes. Blocking these receptors in engram-associated astrocytes using a peptide caused mice to freeze less during recall, indicating that interfering with noradrenergic signaling destabilizes the memory of fear conditioning.
By contrast, overexpression of one of the noradrenergic receptors in astrocytes did not alter freezing activity across two consecutive days. To test if this result reflected some ceiling on behavioral response to fear conditioning, the researchers repeated the experiment using a weaker foot shock. This time, mice with overexpressed noradrenergic receptors froze more on the second day of recall.
These mice also froze in environments other than the foot shock cage after a strong foot shock, suggesting that excess stabilization can cause the overgeneralization of memories. This finding may have implications for understanding post-traumatic stress disorder, the researchers argue. But the researchers boosted noradrenergic receptors in all amygdala astrocytes, not just engram-associated ones; more refined experiments are still a goal of the lab, Nagai says.
T
he new work “adds to mounting evidence that astrocytes actively participate in memory engrams,” says Benjamin Deneen, professor of neurosurgery at Baylor College of Medicine, who was not involved in the new work. His own lab reported interactions between learning-associated astrocytes and neurons in hippocampal engrams in a Nature paper last year.By contrast, the new study found very little astrocytic FOS in the hippocampus after fear conditioning. The researchers reasoned that because Deneen’s lab did not habituate mice to the foot shock cage before conditioning, the hippocampal activity his lab saw could reflect contextual novelty rather than solely learning. Indeed, when Nagai and his colleagues placed mice into a novel environment right before fear conditioning, it produced hippocampal astrocytic FOS activation more in line with Deneen’s results.
Deneen disputes this interpretation, noting that his lab used a different FOS labeling technique and that in his experiments, hippocampal FOS activation in astrocytes subsided after a few hours.
“It’s possible that there’s regional differences between these engram astrocytes,” Deneen says, pointing out that his lab found that activating engram-associated hippocampal astrocytes was sufficient to induce recall in mice, whereas additional experiments would be needed to confirm the same in the amygdala.
Still, there are few established techniques for manipulating or measuring astrocyte activity, Deneen says. “It’s really hard to study [astrocytes] because they’re not electrically active. They sort of keep their secrets, so we don’t really know what to measure.”
These challenges make the astrocyte field “a mess,” Deneen adds, though he says he remains optimistic. “We’re going to learn a lot in the coming decade about the diverse roles that these cells play in active brain circuits.”
With additional reporting by Claudia Lopez Lloreda.
Recommended reading

To persist, memories surf molecular waves from thalamus to cortex

‘How to Change a Memory: One Neuroscientist’s Quest to Alter the Past,’ an excerpt
Expanded view of hippocampal function comes into focus
Explore more from The Transmitter