Machine learning spots neural progenitors in adult human brains
But the finding has not settled the long-standing debate over the existence and extent of neurogenesis during adulthood, says Yale University neuroscientist Juan Arellano.
Neural progenitor cells exist in the adult human hippocampus all the way into old age, a new transcriptomics study published today in Science suggests.
The results strengthen the claim that adults can form new neurons, according to the team behind the work. But not everyone is convinced that the study shows progenitors are prevalent enough in adulthood to really matter.
“Look, there might be something,” says Juan Arellano, a research scientist in neuroscience at Yale University who was not involved with the study. But the cells seem to be rare, because the team could not identify them without the help of a machine-learning algorithm, he adds. “Are they really so relevant in the circuit?”
Although the researchers did not quantify the number of cells in their study, newborn neurons are highly excitatory and plastic, so they might still contribute functionally even if there are few, says study investigator Ionut Dumitru, research specialist in Jonas Frisén’s lab at the Karolinska Institutet.
Proliferating neurons in adults were first documented in a 1998 study that used a synthetic nucleoside to track newly synthesized DNA in newborn cells. Subsequent work involving carbon dating, lineage tracing and tissue-staining techniques bolstered the idea that people can continue to produce new neurons after childhood.
But other studies that stained for cellular markers of neurogenesis suggest that few neurons are born in adults, and the rate of neurogenesis declines dramatically during the first few years of life. These results led some researchers in the field to question the extent and role of neurogenesis in the adult brain, says Shawn Sorrells, assistant professor of neuroscience at the University of Pittsburgh, who conducted some of these cellular marker studies but was not involved with the new one.
Still, the techniques used to track the cellular markers, which were identified mainly in rodents, might not be sensitive enough to detect human progenitor cells, a 2023 study argued. The advent of transcriptomics presented an unbiased way to capture rare cell populations without having to rely on these markers, says Paul Lucassen, professor of brain plasticity at the University of Amsterdam, who was not involved with the new study. By combining this approach with machine learning, researchers previously identified immature granule cells in adult brains.
The researchers behind the new study added another step: They isolated proliferating cells from the brains to make it easier for them to find progenitors if they exist.
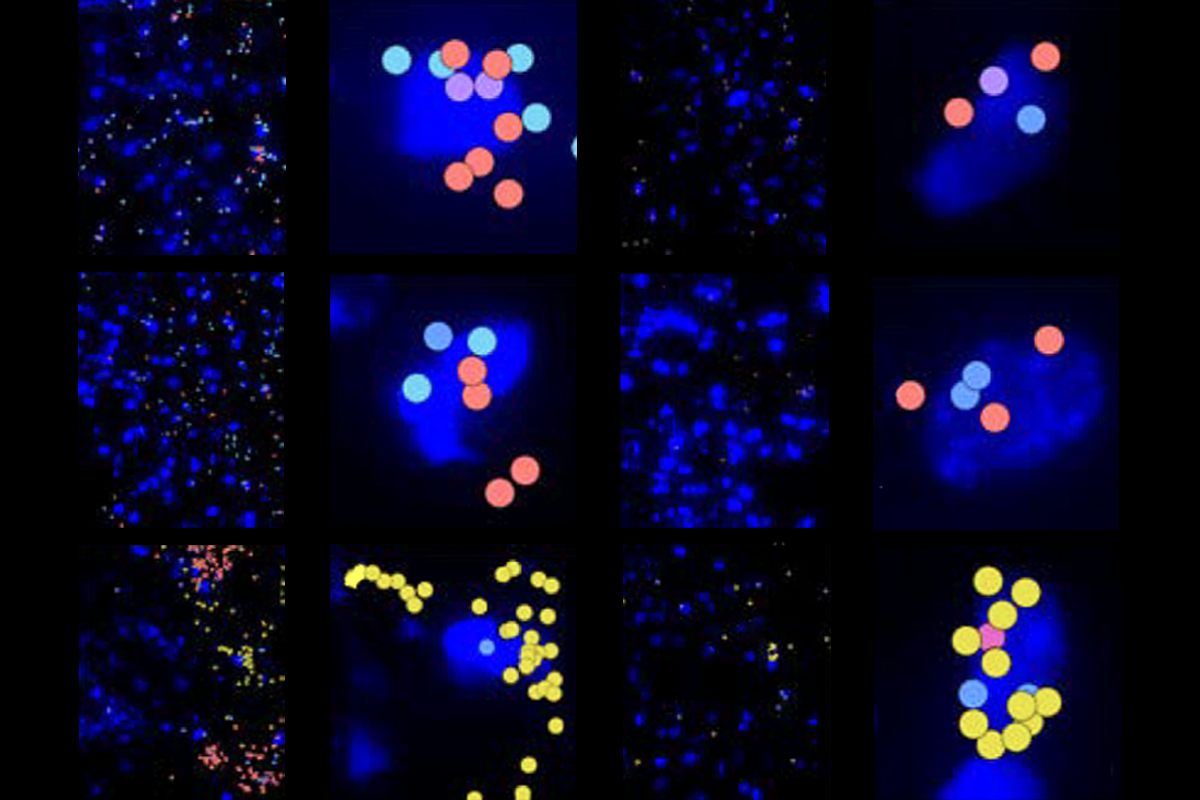
They used single-nuclei RNA sequencing to profile the neural progenitor cells from the hippocampus of six people aged 5 years or younger. A machine-learning algorithm trained on the transcriptomic signature of those cells then identified the signature of progenitor cells—predicted to be a mix of neural stem cells, immature neural progenitor cells and neuroblasts—in sequencing data from the dentate gyrus from nine people aged 20 to 78 years.
“I think we can all agree that in the adult human brain there are cells with these features,” says Evgenia Salta, group leader at the Netherlands Institute for Neuroscience, who was not involved with the study.
W
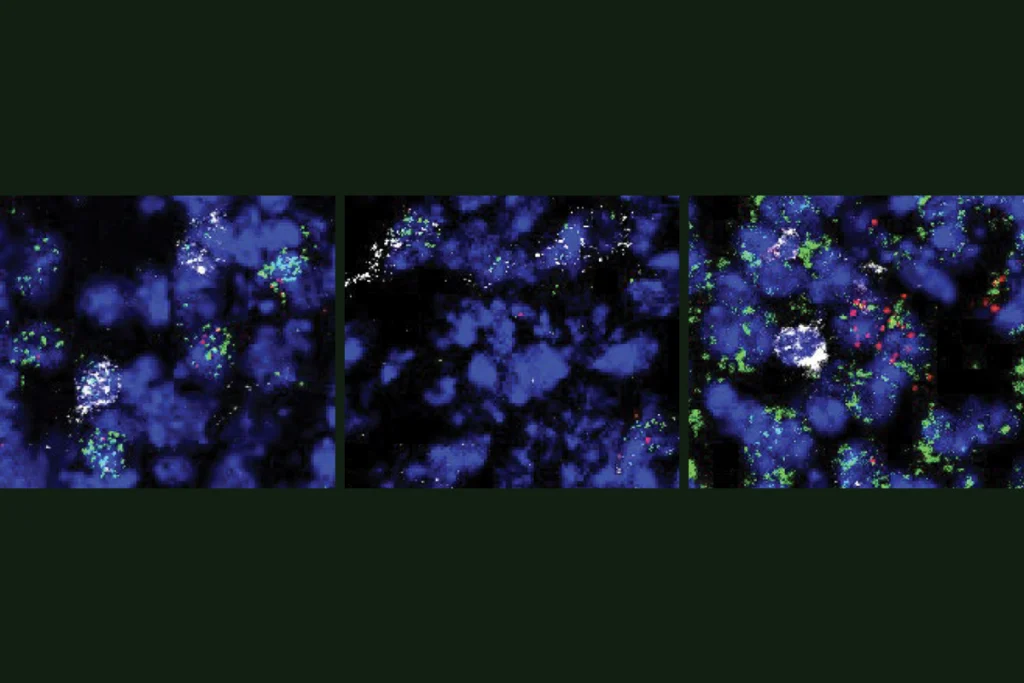
hen Frisén and his team compared their data to published datasets, they found that the adult neural progenitor cells they identified had similar expression profiles to their corresponding cells in other species, including rhesus macaques, pigs and mice. The levels of neural progenitor cells varied from person to person, and five additional adults had no detectable cells.The three types of neural progenitor cells were enriched for a variety of markers, the researchers found using a transcriptomics platform called Xenium that allows for the staining of 300 markers, which helped them map the spatial distribution of the cells. The progenitors, which localized to the dentate gyrus, did not express markers of non-neurogenic cell types, such as microglia and astrocytes.
Doublecortin, used often to identify neuroblasts and immature neurons, was broadly expressed across cell types in the samples, suggesting that it alone is not sufficient to identify these cells and that “you need a lot of markers to define a progenitor cell,” says Marta Paterlini, a researcher in Frisén’s lab who conducted the work.
With these technologies, “we are able to find the full molecular profile of these cells,” Salta says. “Now it seems that we have the full trajectory, for the first time, also in the adult human brain.”
And the results confirm “that we will never be able to find this one cell-type-specific marker for the human neurogenic populations, as we know from the mouse brain, and this is very OK,” Salta adds. “That would make our life as researchers, of course, much easier. But the human brain is a complicated organ, and it’s a good time to acknowledge the complexity.”
T
he expression profile of the adult progenitor cells identified in the study overlaps in part with other cell types, such as astrocytes and immune cells, so Arellano says he wonders if they are indeed neural progenitor cells. If they are progenitor cells, it remains unclear if this reflects bona fide adult neurogenesis, because they could have originated during embryonic development and only matured later, Salta says. “This is still something we don’t know for sure, but the cells are there.”If neurogenesis occurs in adults, it is likely so rare and sparse that it is irrelevant for the brain, according to Sorrells. And the fact that the team had to use machine learning to find progenitor cells and did not detect them in 2 of 4 adolescents they studied, nor in 5 of the 14 adults, signals that the neurogenic process is variable and limited, Arellano says. What’s more, many of the brain donors had serious psychiatric conditions or neurological injury, which can influence the level of dividing cells, Sorrells says.
But that variability could reflect real biological variability and merit further study, Salta says. For example, one of the people with the highest levels of immature neural progenitor cells had epilepsy, which has been linked to aberrant neurogenesis. “Maybe individual variability has something to teach us when it comes to the phenomenon of neurogenesis in the adult human brain,” Salta says. “It’s possible that this is a very sensitive process that is really sensitive to many extrinsic and intrinsic factors.”
Recommended reading
New autism committee positions itself as science-backed alternative to government group

Two neurobiologists win 2026 Brain Prize for discovering mechanics of touch
Shifting neural code powers speech comprehension
Explore more from The Transmitter
Novel neurons upend ‘yin-yang’ model of hunger, satiety in brain
Imagining the ultimate systems neuroscience paper