Immune cells block pain in female mice only
Regulatory T cells in the spinal meninges release endogenous opioids in a sex-specific manner, new work shows.
A class of immune cells releases endogenous opioids to quell pain in female—but not male—mice, according to a new study. This sex-specific mechanism for pain modulation at the spinal cord may help explain why women are disproportionately diagnosed with chronic pain, the researchers say.
Many people with autoimmune conditions experience increased levels of pain, and the immune system is thought to shape pain sensation, says Sakeen Kashem, assistant professor of dermatology at the University of California, San Francisco, who co-led the work. But exactly how immune cells communicate with the brain has been unclear, he says.
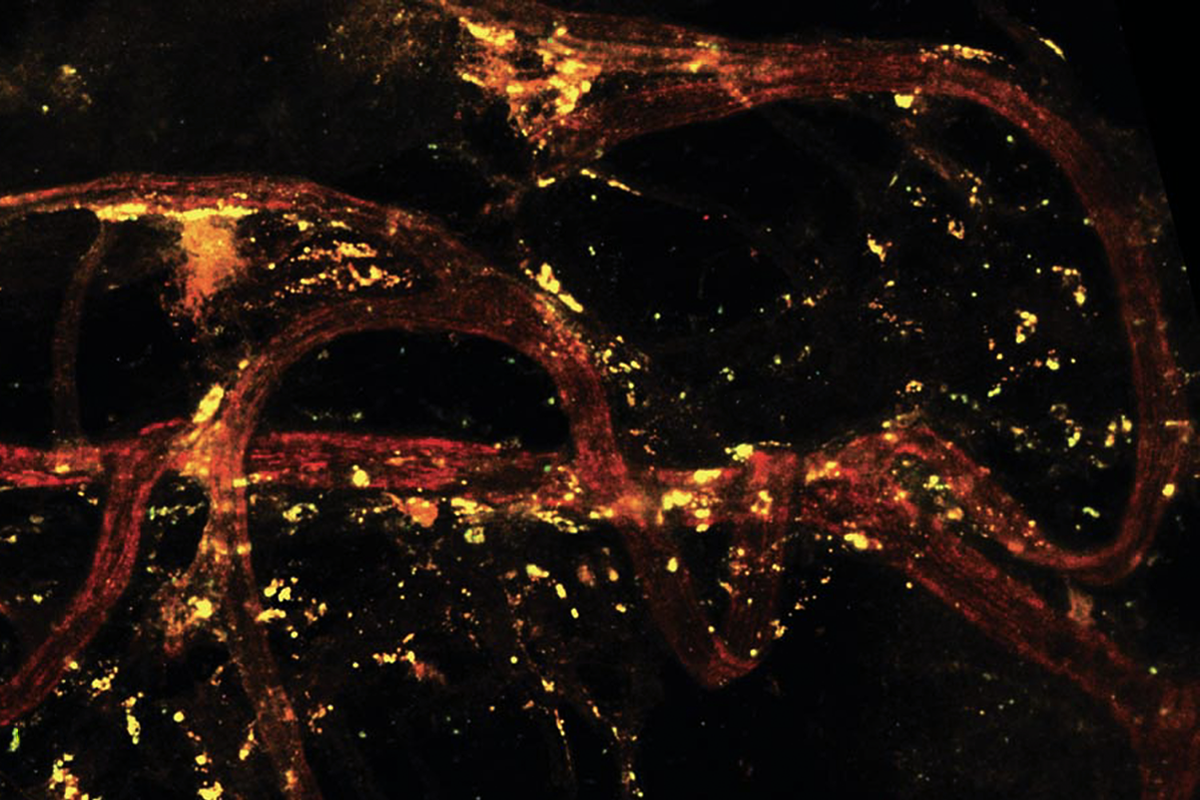
Those signals—at least in female mice—come from meningeal regulatory T cells, which are located in the fluid surrounding the spinal cord and help tamp down the body’s inflammatory response after injury, the new study shows: Aided by estrogen and progesterone, these cells release enkephalins, endogenous opioids that bind to neuronal opioid receptors.
“They make a connection that has really been missing in our understanding of all this physiology,” says Asya Rolls, professor of neuroimmunology at Tel Aviv University, who was not involved in the work.
The result adds to a growing understanding that the immune system does not just induce pain but can suppress it as well, says Geoffroy Laumet, associate professor of physiology and neuroscience at Michigan State University, who was not involved in the study. And it helps confirm the immune system’s sexual dimorphism, which may contribute to higher levels of chronic pain conditions—such as fibromyalgia and migraine—in women than in men, he says: “Sex differences in immune cells result in sex differences in pain.”
The findings also point to the importance of studying both male and female mice, Kashem says. “If we had only looked at male mice, we would never have found this,” he says. “It was a lot of effort to use both sexes. It’s more expensive. But at the same time, it leads to a greater understanding.”
K
ashem and his colleagues injected both male and female mice with a toxin that depletes regulatory T cells in the spinal cord’s meninges—after which female mice, but not male mice, became hypersensitive to pain stimuli. And when the researchers mimicked a sciatic nerve injury in the animals’ hind legs, only the female mice showed high pain sensitivity when the leg was touched. Boosting the levels of regulatory T cells in the spinal meninges restored the female mice’s pain sensitivity to normal levels but had no effect on the male mice, the work shows.To investigate the cause of this sex difference, the team ran the same tests in mice that have male (XY) chromosomes and female gonadal hormones, as well as mice with female (XX) chromosomes but lack female gonadal hormones. Mice that produced estrogen and progesterone exhibited pain hypersensitivity after depletion of meningeal regulatory T cells—suggesting that these hormones are involved in how the immune cells shape an animal’s pain experience. And female mice with meningeal regulatory T cells engineered to not release enkephalins were similarly hypersensitive to pain. The findings were published last month in Science.
Enkephalins bind to delta opioid receptors in primary sensory neurons, where they limit pain sensitivity. “For the first time, we’ve now figured out that cells in the meninges can talk to the peripheral nervous system,” Kashem says.
The mechanism described in the new study reveals how regulatory T cells mediate mechanical pain, the kind conveyed through a tactile stimulus. But other forms of pain—such as heat pain—may be modulated through different pathways, says study investigator Élora Midavaine, a postdoctoral researcher in Allan Basbaum’s lab at the University of California, San Francisco.
Moving forward, it will be interesting to see how well this mechanism generalizes across different types of pain and different parts of the body, says Gregory Dussor, professor of neuroscience at the University of Texas at Dallas, who was not involved in the work. Given the stark sex differences seen in chronic migraine pain, for example, “I would love to see a follow-up study from this group or someone that looks at whether or not this is similar in the cranial meninges.”
And whether the findings translate to people also remains to be seen. People without regulatory T cells are known to experience increased levels of pain, Midavaine says. But more work is necessary to determine whether the same mechanism holds up across species, she says.
Still, the work highlights the complex relationship between the immune system and the brain, Midavaine says. The regulatory T cells that shape pain sensing in female mice “are not even in the spinal cord; they’re really in those border tissues. So we have to look at the central nervous system as being more widely modulated than what cell types are directly in there.”
That offers a lesson for neuroscientists, Laumet says. “Neurons do not work in isolation. They can usually be influenced by immune cells around them, and that’s something that we really need to take in consideration.”
Recommended reading
Human brain may anticipate looming contagion

Rethinking mental health: The body’s impact on the brain
New autism committee positions itself as science-backed alternative to government group
Explore more from The Transmitter
Novel neurons upend ‘yin-yang’ model of hunger, satiety in brain
Immune cell interlopers breach—and repair—brain barrier in mice
