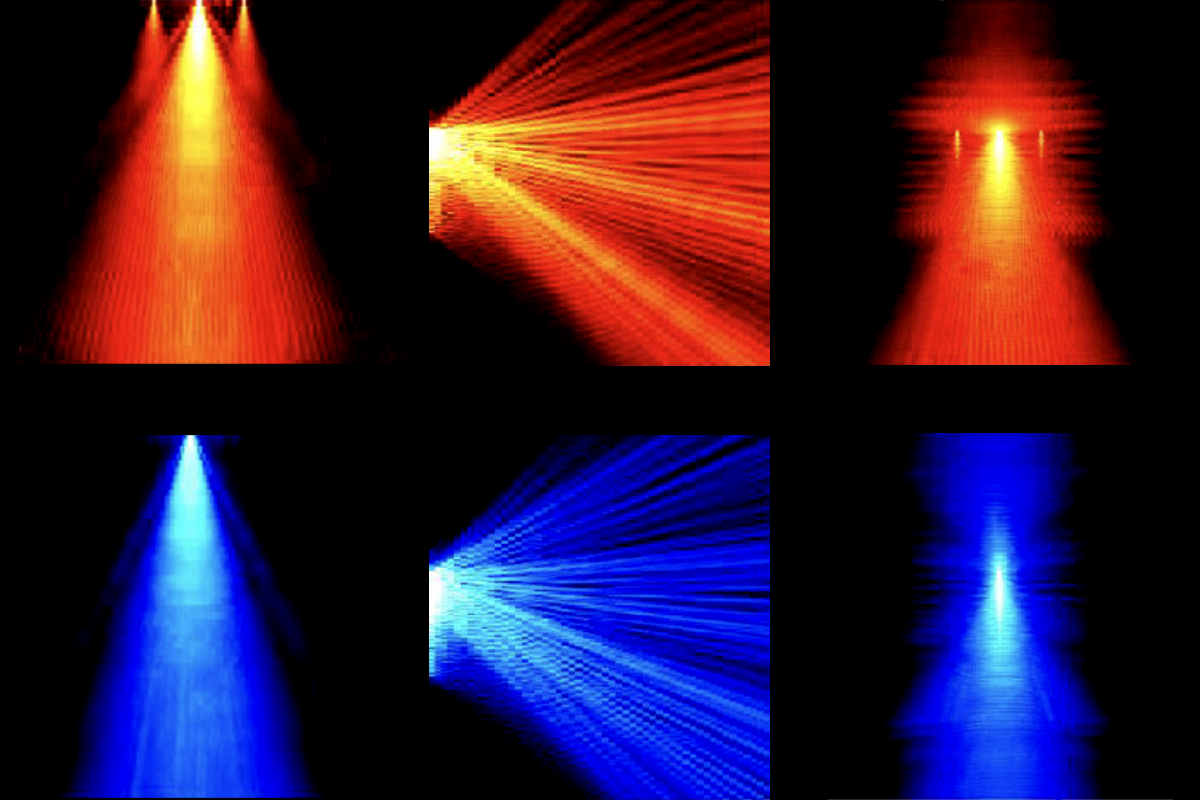
A new probe called Neuropixels Opto emits light to modulate thousands of neurons optogenetically and also measures their resulting shifts in electrical activity across 960 recording sites. Researchers describe the probe and its application in a preprint posted on bioRxiv in February.
This kind of combination device is crucial because “to understand the mechanisms underlying computation, you need to both perturb and record,” says Ilana Witten, professor of neuroscience at Princeton University, who was not involved in the work.
Previous iterations of such instruments have fewer recording sites, and many use micro-LEDs that can become hot and damage tissue when trying to provide a lot of light, says Matteo Carandini, professor of visual neuroscience at University College London and one of the lead researchers of the work.
By contrast, Opto probes pair a Neuropixels multielectrode array with a photonic waveguide that channels light from an external laser, thereby avoiding the heating issue. The resulting device can provide light from 28 emission sites and record from different areas of the brain just as well as electrodes not used with the photonics, Carandini and his colleagues found in mice. It can also record at a larger scale and with more precision than previous appliances—and from deeper brain structures, such as the striatum, which have been challenging to target with optogenetics because of the limited light penetrance.
This tool is “not only democratizing a relatively challenging method but also making it much better,” Witten says. They are “improving the quality of the results and making it easy to use.”
O
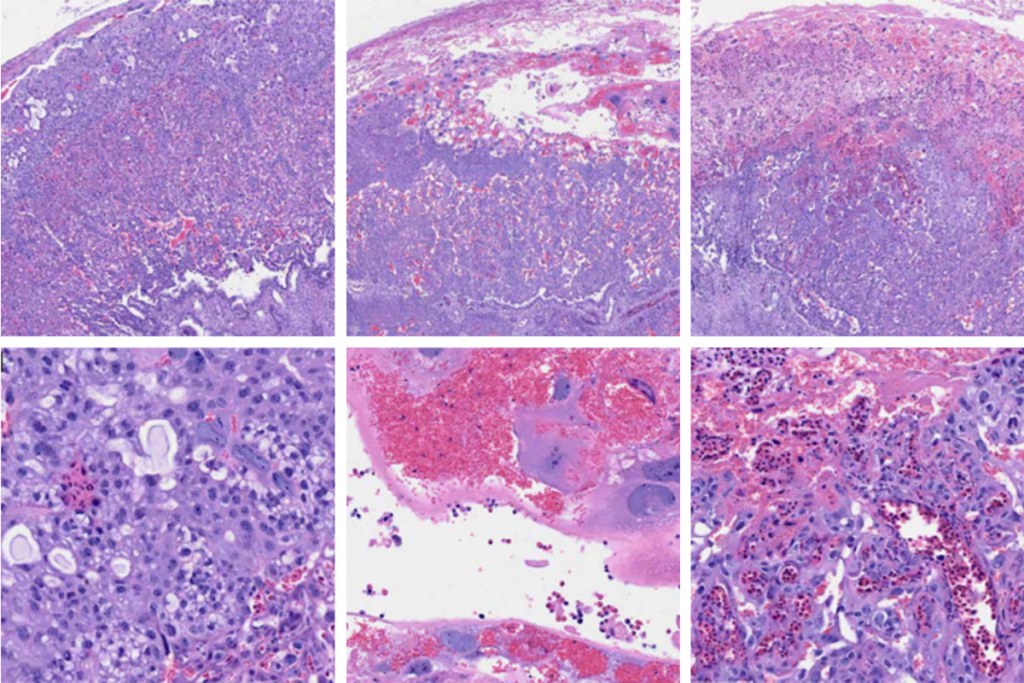
ne of the most useful potential applications of Opto probes is optotagging, Witten says. Optotagging capitalizes on the light-sensitive proteins used in optogenetics, but instead of using those proteins to activate or silence a neuron, it uses them to identify, quantify and characterize cells of interest in vivo.In the new work, the team tagged 294 cells in the mouse striatum, globus pallidus and the midbrain reticular nucleus, efficiently activating them and tracking their electrical signals. The Opto probe was able to tag cells more effectively than other optotagging approaches, Witten says. Their data is “some of the best phototagging data I’ve seen.”
“We have a bigger toolbox now to investigate these subtypes of cells, even if they’re intermixed in space,” says Edvard Moser, group leader at the Norwegian University of Science and Technology who was not involved in the creation of the device.
In addition to optotagging, researchers could also use Opto probes to interrogate brain-cell populations and networks. “This will be a very powerful method to both identify cells, but also to inhibit or stimulate them in order to test functions within the circuit,” he says.
In a test run, Carandini’s team turned on one light emitter at a time at different locations throughout the mouse visual cortex and saw activity only in that small region, proving that they could manipulate specific areas with high spatial resolution. In part because of the short distance between the stimulation site and the array, the Opto probe lets researchers “stimulate much more precisely,” Moser says.
This precise stimulation opens the door to begin to modulate particular regions to study different circuits. “That is the dream,” Carandini says.
Carandini and his team are working to add even more light emitters to the probe to boost its power, among other improvements, he says. In the future, perturbing neurons and recording them separately might become a thing of the past, he adds. “It’s possible that we will look back at the experiments that we’ve been doing now, in which we either record from a lot of neurons or we stimulate, and realize that we need both.”