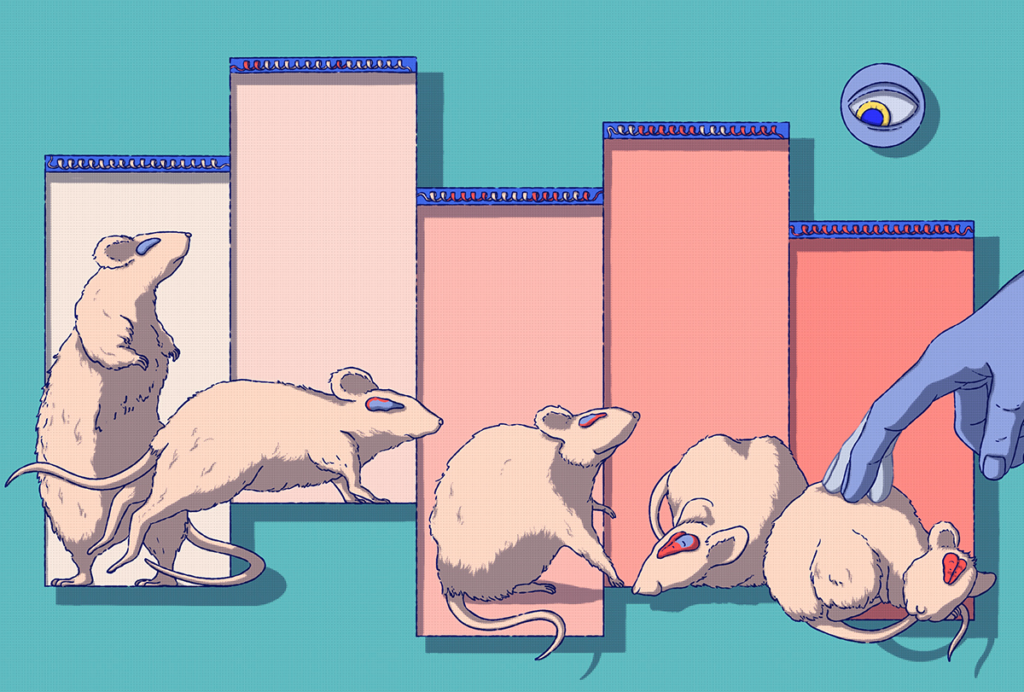
Monkey with mutation in top autism gene shows social problems
The first monkey with a mutation in SHANK3, a top autism gene, is nearly 3; it spends its days circling its cage rather than interacting with other monkeys.
The first monkey with a mutation in SHANK3, a top autism gene, is nearly 3 years old1. It spends its days circling its cage rather than interacting with other monkeys.
The monkey lacks SHANK3 in only some of its cells — and yet shows traits reminiscent of autism, including social disinterest and repetitive behaviors.
Roughly 2 percent of people with autism carry a mutation in SHANK3. Researchers have engineered 14 lines of mice with this mutation, but none of them reliably replicate the behaviors seen in autistic people.
Monkeys have an edge over mice both for recapitulating human behavior and for testing drugs to treat those behaviors, says lead researcher Yong Zhang, professor of genetics and developmental biology at the Chinese Academy of Sciences in Beijing.
“Our main goal is to test and validate drugs,” Zhang says. “If [a drug] works in monkeys, then it would be a good argument to try it in humans.”
Zhang and his colleagues hope to use their monkey to breed more animals with the mutation, allowing for more controlled experiments. So far, they have been able to show that two weeks of treatment with the antidepressant fluoxetine, which is also prescribed for some forms of anxiety, eases some of the monkey’s unusual behaviors.
“The autism-like deficits in behavior are striking,” says Michael Platt, professor of psychology at the University of Pennsylvania in Philadelphia, who was not involved in the study. “The fact that the authors could improve these anomalies with fluoxetine is remarkable.”
Monkeying around:
Zhang’s team used the gene-editing tool CRISPR to snip out a portion of SHANK3 in single-cell monkey embryos. Last year, they reported that this technique yielded three monkeys from 116 embryos implanted into 37 surrogate mothers; two of the monkeys died before or at birth.
The third monkey was 26 months old — the equivalent of human childhood — when the researchers wrote up their results. Now approaching sexual maturity, the monkey is thriving, Zhang says, but its unique behaviors prevent it from forming attachments with peers. For example, the mutant monkey did not vocalize until 18 months of age, whereas three control monkeys did so immediately after birth.
The researchers video recorded the mutant and the control monkeys twice a day for 30 minutes, for six days. Three scientists who did not know which monkey was which scored the animals’ behaviors.
In one video, the mutant monkey climbs up the wall of its cage, across the roof, and down the other side — over and over again. Meanwhile, one of its typical peers investigates a ball on a chain, chews the chain for a while, peeks out of its cage and returns to bounce the ball.
Overall, the mutant monkey explores its cage less than the controls and spends more time engaging in persistent behaviors with no apparent purpose, such as pacing or rocking. For example, for every hour of recording, it spent roughly 13 minutes on average repeating one behavior; in that same period, one of the controls engaged in no repeated actions, one did so for less than 2 minutes and the third for roughly 7 minutes.
When the researchers introduce an especially social fourth monkey to the mutant monkey, the latter climbs repeatedly over the roof of the cage with its tail held high — a sign of anxiety. It spends only about 1 minute, on average, of the 30-minute sessions with the social monkey. By contrast, the typical monkeys spend between 4 and 25 minutes with their social peer, tussling and joining forces to investigate a chain in the cage.
“The data indicates the [mutant] monkey is a good model for autism,” says Yongchang Chen, professor of developmental biology at Kunming University of Science and Technology in China, who was not involved in the study.
The researchers gave the mutant monkey and controls up to 2.5 milligrams of fluoxetine per kilogram of body weight, ground into their food, each day for two weeks. During treatment, the mutant monkey circled its cage 86 percent less overall, and socialized for 15 times longer than before. After treatment ended, the monkey returned to its previous behaviors.
High anxiety:
The SHANK3 monkey’s social issues may stem, in part, from its repetitive behaviors.
“The social behaviors are so reduced they are almost nonexistent,” Platt says. “That makes me wonder if it is the hyperdrive on stereotypical behaviors that is reducing time or interest in socializing.” Fluoxetine’s effectiveness suggests that anxiety drives the monkey’s asocial behavior, he says.
In their previous study, the researchers analyzed the brains of the two mutant monkeys that did not survive. The monkey that died before birth had more brain cells lacking SHANK3 than the one that died soon after birth; the surviving mutant probably has even fewer cells that lack SHANK3, the researchers say.
The surviving monkey is smaller than its typical peers, but its head size is in the normal range. Brain scans reveal no differences in brain structure between the mutant monkey and controls.
For the mutant monkey to pass down its mutation to its baby, its germ cells must carry the mutation; the researchers are investigating whether that is the case.
References:
- Tu Z. et al. Hum. Mol. Genet. Epub ahead of print (2018) PubMed
Recommended reading

Sex bias in autism drops as age at diagnosis rises
Microglia implicated in infantile amnesia
Explore more from The Transmitter
Neuroscience needs single-synapse studies