Ramping up cortical activity in early life sparks autism-like behaviors in mice
The findings add fuel to the long-running debate over how an imbalance in excitatory and inhibitory signaling contributes to the autism.
A temporary increase in neuronal activity in the cortex of newborn mice leads to social deficits in adulthood, according to a new preprint. Those adult rodents also show changes in brain electrical activity, gene expression and connectivity that are reminiscent of autism.
The analysis lends support to a prominent hypothesis of autism’s origins, which holds that the condition can arise from an excess of excitatory signaling or insufficient inhibitory signaling in the brain, the study investigators write in their paper.
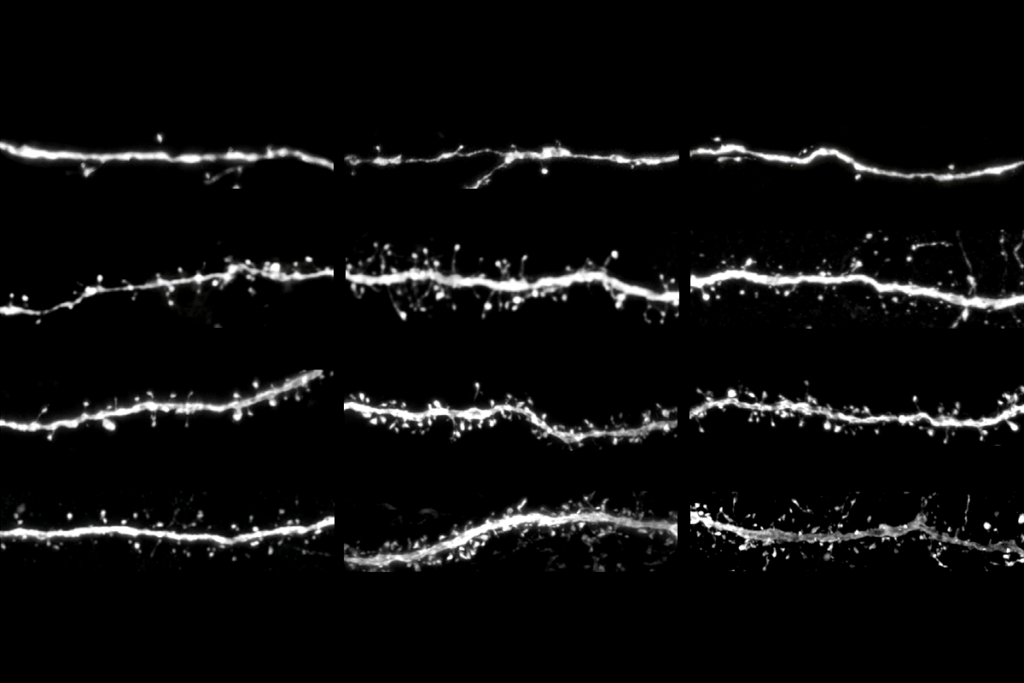
Over the years, support for this signaling imbalance hypothesis has come from other studies in mice and observations that some people with autism have seizures or display excess neuronal activity in electroencephalography (EEG) recordings relative to people without the condition. Postmortem analysis suggests autistic people have more excitatory synapses in the prefrontal cortex than non-autistic people. But determining causality and the role of inhibitory signaling has been difficult.
In contrast with most earlier work, the new study “really underscore[s] a different way of looking at excitation-inhibition imbalance, which is looking at it during development as a cause of subsequent changes in brain function that could be associated with autism,” says Vikaas Sohal, professor of psychiatry and behavioral science at the University of California, San Francisco, who was not involved in the work.
The study was posted on bioRxiv last month.
T
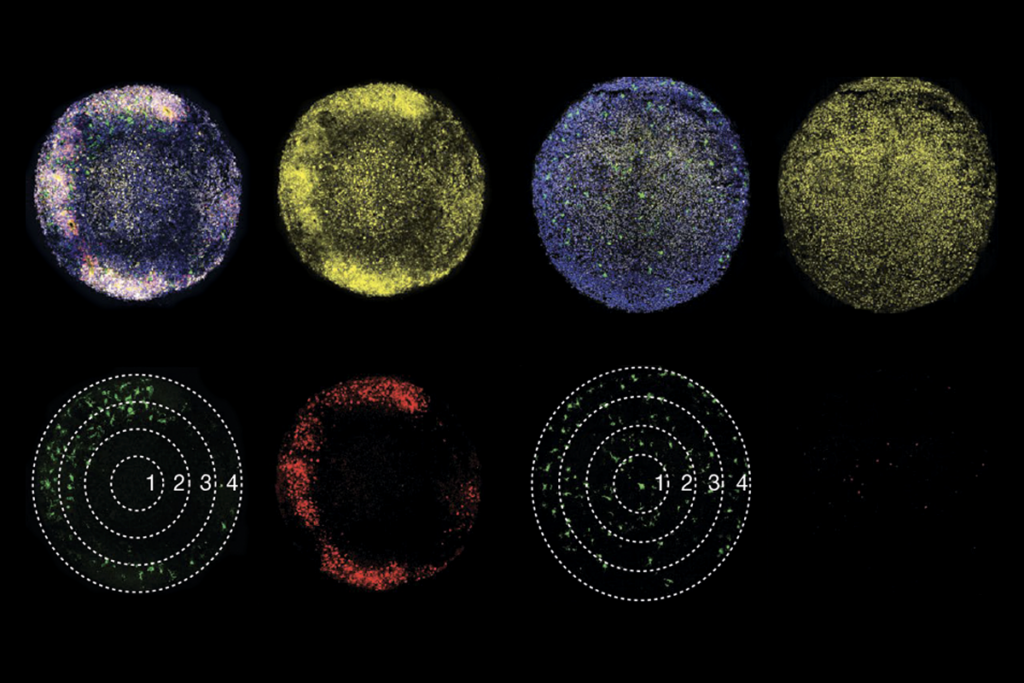
he new study employs chemogenetics, a method that involves mice genetically engineered to express receptors for certain drugs in specific brain cells or regions. Dosing the mice with such drugs at specific times enables more finely targeted manipulations of brain activity than have been possible in the past.In this case, a drug was used to increase the activity of excitatory pyramidal neurons in the cortex and hippocampus of mice for the first two weeks of life, a window that roughly corresponds to late gestation and early infancy in people. Across the cortex in these mice, neurons exhibit increased rates of spontaneous firing.
Later on—in late infancy, adolescence, young adulthood and adulthood—the chemogenetically manipulated mice are less sociable than control mice, the study investigators found.
The effects of the chemogenetic manipulation are specific to social behavior. A battery of tests turned up no changes in sensory, motor or cognitive functions in the mice. “That was really one of the most surprising findings in our work,” says study investigator Alessandro Gozzi, senior researcher at the Istituto Italiano di Tecnologia.
Timing is also crucial. Inducing a temporary increase in cortical activity in adolescent mice has no effects on their social behavior, according to other experiments reported in the preprint. “It’s not just making circuits hyperexcitable at any time that’s important,” says Takao Hensch, professor of neurology and molecular and cellular biology at Harvard University, who was not involved in the work. “It’s during this critical window early in life.”
Work from Hensch’s lab previously showed that there is a critical period for multi-sensory integration and repetitive behavior in mice that occurs shortly after the time of the chemogenetic intervention in the new study. “Varying the timing a little finer might extract different aspects of the autism phenotype,” Hensch says.
As adults, mice that were treated chemogenetically in infancy have excess activity in the prefrontal cortex, as shown by EEG recordings. Two drugs that enhance inhibitory signaling, R-baclofen and bumetanide, each rescue social deficits in the mice.
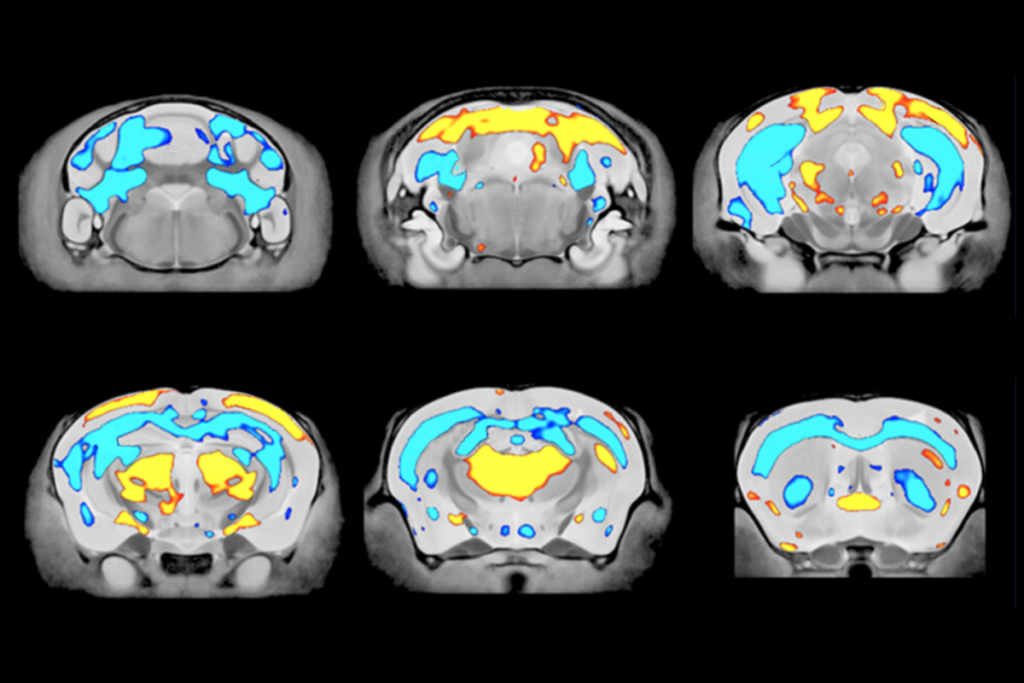
In resting-state functional MRI (fMRI) scans, the mice showed weaker-than-normal connections between the prefrontal cortex and other brain areas, especially those involved in social behavior.
T
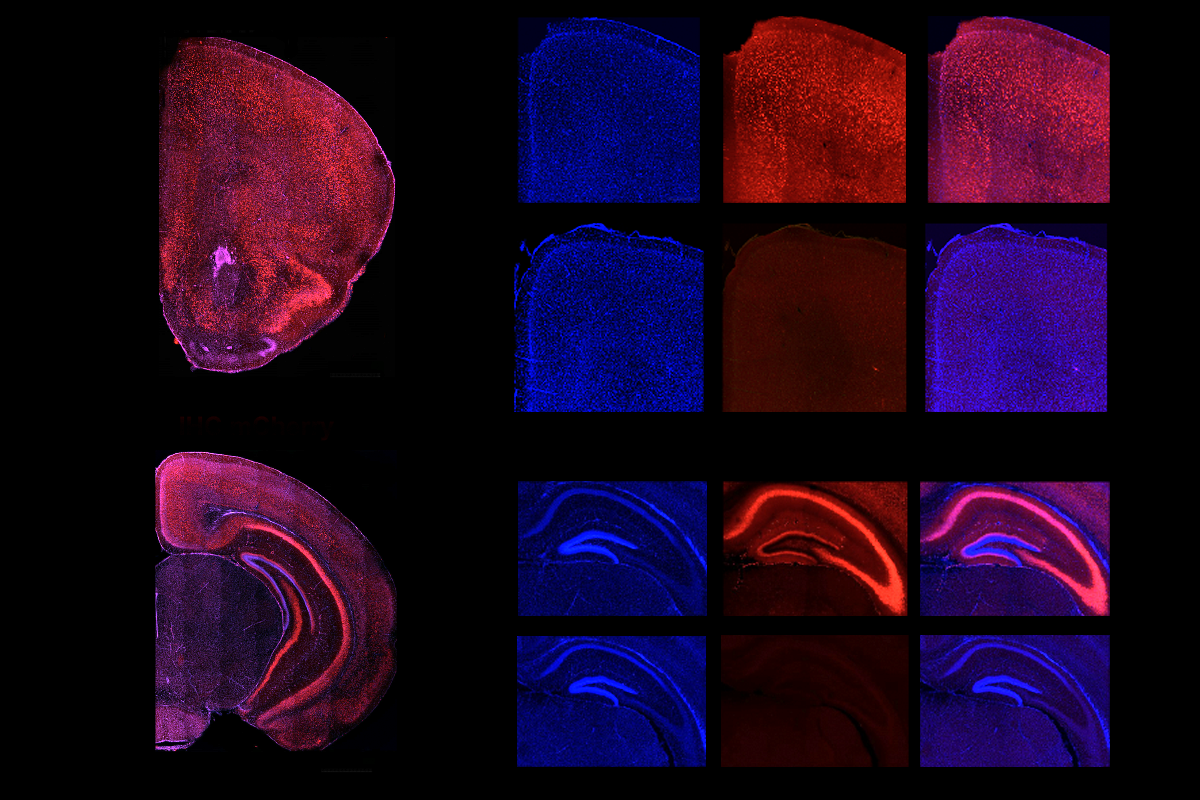
he chemogenetically treated mice also show alterations in brain expression of 530 genes, half of which are upregulated and half downregulated compared with wildtype mice. The differentially expressed genes include many that are relevant to autism but not to schizophrenia or bipolar disorder, and many that play a role in synapse formation and function.“That’s very satisfying,” Hensch says of the inclusion of multiple other lines of evidence to back up the behavioral findings.
Stepping back, the study investigators suggest their work offers a definitive answer to how an excitatory-to-inhibitory (E:I) signaling imbalance relates to autism. “I think our data are really clear cut,” pointing to a causal effect of altered signaling on autism-like traits, Gozzi says. “Our work, we believe, settles this long-standing controversy.”
But not all researchers agree. “I don’t think that this experiment proves or disproves the idea of how E:I balance is related to autism,” says Dan Feldman, professor of neuroscience and neurobiology at the University of California, Berkeley.
Excitation and inhibition “are really best defined on the synaptic level,” says Feldman, who previously led mouse research suggesting that a signaling imbalance may be a compensatory mechanism rather than a cause of autism. “What the data most directly show is that creating an early cortex that is too active leads to lasting changes.”
Gozzi contends that his team’s examination of excitation and inhibition at the neuronal population level, further detailed in a 2024 preprint, is “complementary” to the synaptic definition. “Our approach avoids layer- and circuit-specific ambiguities of the synaptic ratio, is directly measurable in vivo and scales naturally” to measures of brain activity such as EEG and fMRI that can be used in people, he says.
Another study from Gozzi’s lab divided 20 different mouse models of autism into two subtypes, depending on whether they exhibited unusually strong or weak connectivity in fMRI scans. These patterns were also seen in a subset of people with autism.
The new work suggests that excess cortical activity early in development underlies the weaker-than-normal connectivity pattern. “What we would like to do now is to try to do the reverse manipulation,” Gozzi says. “We want to suppress excitability during this window and see whether we get the other opposing signature.”
Recommended reading
New autism committee positions itself as science-backed alternative to government group
Astrocytes orchestrate oxytocin’s social effects in mice
Explore more from The Transmitter
Sensory gatekeeper drives seizures, autism-like behaviors in mouse model