How developing neurons simplify their search for a synaptic mate
Streamlining the problem from 3D to 1D eases the expedition—a strategy the study investigators deployed to rewire an olfactory circuit in flies.

The hunt for a soulmate can be hard work—particularly for naive neurons. During development, the cells’ axons snake through burgeoning brain areas in search of the perfect dendrite to form a synapse with. Cell surface proteins serve as molecular identification tags to help axons distinguish “Mr. Wrong” dendrite from “Mr. Right,” according to the chemoaffinity hypothesis.
But there are too many cells and too few cell surface proteins for this to be the only strategy, says Claude Desplan, professor of biology and neural science at New York University. “There is no way you can find your partner in a big mess of many different thousands of types of neurons. So you do need to reduce the issue.”
Neurons in the developing Drosophila antennal lobe simplify the problem by turning a 3D search into a 1D one, according to a paper published 1 May in Science.
In this brain region, 50 types of olfactory receptor neurons link up with 50 types of neurons that project to a sensory integration hub called the mushroom body; each synapse type bunches together inside the lobe to form its own distinct glomerulus. The axons of olfactory receptor neurons do not search the entire structure for their postsynaptic partner. Instead, the projection neurons inside the lobe send their dendrites to meet axons traveling along the surface. Once the two join up, they descend to their proper place in the lobe, imaging experiments show.
“Axons don’t need to delve deep. They only need to survey the surface in order to find their target,” says the study’s principal investigator, Liqun Luo, professor of biology at Stanford University. To make matters even simpler, the axons stick to a narrow, genetically determined trajectory, Luo says. Cortical regions may achieve a similar simplification through columns and layers: Axons travel to a certain brain region and then plunge to a particular depth, Luo suggests.
Genetically altering these trajectories precludes the olfactory receptor neurons from finding their proper mate, additional experiments show. Dendrites from the postsynaptic cell still wait for their partner at the surface, but “they will be sitting there waiting forever,” Luo says. Some cells “are still sticking their dendrites out” in adulthood, and in at least one case the team observed, a cell eventually matched with another partner.
The work “provides a really nice, elegant demonstration” that “axons are limiting their sampling,” says Julie Lefebvre, senior scientist of neurosciences and mental health at the Hospital for Sick Children and assistant professor of molecular genetics at the University of Toronto, who was not involved in the study.
L
uo and his team applied this “dimensionality reduction” principle to rewire an olfactory circuit, according to a pair of preprints posted on bioRxiv in March. Knowing that a neuron encounters only a handful of glomeruli along its preset path rather than all 50 “helped make the rewiring much simpler,” Luo says. “It’s much easier to conceive that you can solve that problem.”Several cell surface protein dynamics must change to swap synaptic partners, Luo says: You must sever the connection between the original partners by breaking attraction and increasing repulsion, disrupt the repulsion between the new partners and add attraction. “It reminds me a little bit of the multiple hits idea in cancer,” says Peter Robin Hiesinger, professor of neurobiology at Freie Universität Berlin, who was not involved in the study.
The team compared the transcriptomes of pre- and postsynaptic neurons from 13 synaptic pairs in the antennal lobe and identified sets of attractive and repulsive cues. If a molecule and its partner are highly expressed on both sides of a synapse, they are likely an attractive cue, the team reasoned, whereas sets of molecules with inverse expression patterns are likely repulsive. “It took a long time to break the combinatorial code,” Luo says.
But break it they did. The team rewired a circuit involved in detecting a male pheromone by manipulating the expression of attractive and repulsive cues using cell-type-specific genetic tools. The original partnership inhibits male-male courtship, but the new partnership promotes it. Male flies with the rewired circuit “exhibited vigorous chasing and courtship activities, sometimes forming a courtship chain in which a male attempted to court the male in front of him while being courted by another male behind him,” the team wrote in the preprint.
The work is the “first to have shown—beautifully, in the olfactory system here—that by taking a composite of cell surface manipulations, you can predictably rewire,” Hiesinger says. That predictability is what makes the rewiring so compelling, says Desplan, who was not involved in the work. “It’s very easy to disrupt” a circuit without knowing what you are changing and how, he adds.
The studies demonstrate the importance of studying development as it unfolds rather than just perturbing a gene or process and focusing on how it changes the outcome, Hiesinger says. “There are developmental processes that happen that you would never know about if you only looked at the outcome,” such as how fast an axon grows, the way it branches and the shape and maturity of other neurons it encounters.
These factors limit the possible mates for a developing neuron, Hiesinger says. It’s like dating, he adds: You can only date someone you meet, and you won’t meet everyone. You have to meet at the right place and time. And at “the moment of choice,” you must both be mature, have enough time to interact and at least “not be repulsed” by each other, but it’s even better to be attracted, he says.
The moment of choice is not addressed by the current studies, Hiesinger says. It’s not clear from the rewiring preprints if cell surface proteins act only as long-distance cues to guide neurons in the right direction or if they are also involved in actually forming the synapse, he adds.
If axons end up in the wrong place, they might form a synapse with whichever cell is close to them, Hiesinger says, an idea known as synaptic promiscuity. Examining if misdirected axons form synapses with inappropriate partners would make a compelling follow-up, Lefebvre says. “My guess would be that they probably don’t—that there are other mechanisms in place to ensure that they will not make a synapse onto the wrong target.”
Overall, the new body of work “reinforces the importance of developmental strategies to reduce the complexity of synaptic wiring,” Lefebvre says, and shows that there are “cellular steps along the way that simplify and reduce the number of choices.”
Recommended reading
Cell atlas cracks open ‘black box’ of mammalian spinal cord development
Waves of calcium activity dictate eye structure in flies
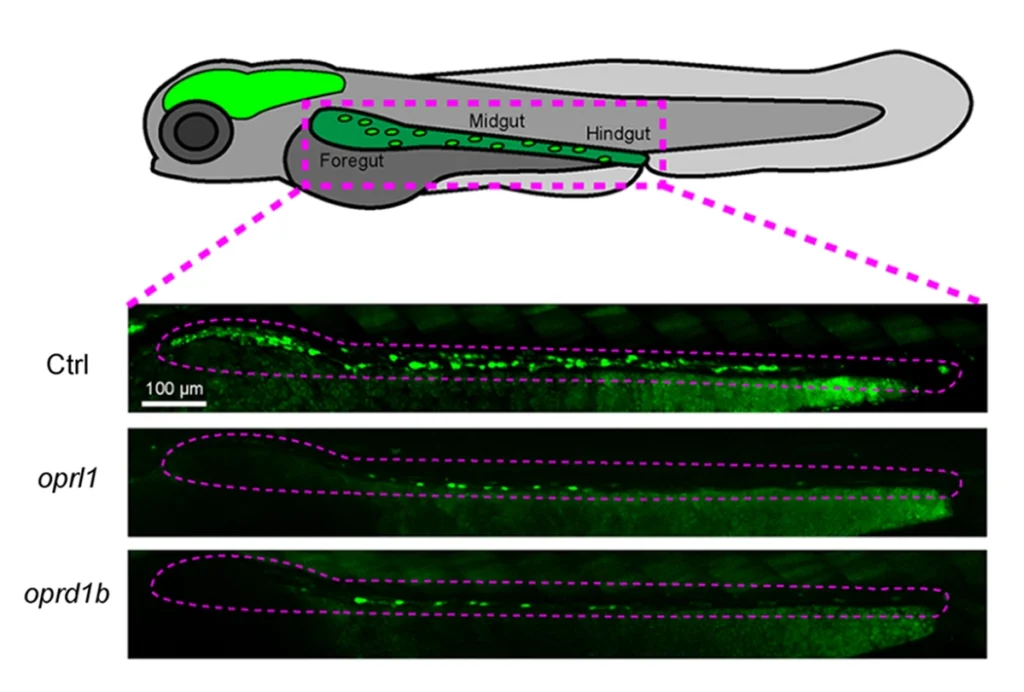
Opioid receptors may guide formation of gut nervous system in zebrafish
Explore more from The Transmitter
Novel neurons upend ‘yin-yang’ model of hunger, satiety in brain