New dopamine sensor powers three-color imaging in live animals
The tool leverages a previously unused segment of the color spectrum to track the neurotransmitter and can be used with two additional sensors to monitor other neurochemicals at different wavelengths.
A new sensor makes it possible for the first time to simultaneously track dopamine and up to two additional molecules in the brains of living animals. The sensor, dubbed HaloDA1.0, uses a novel dopamine-tagging system that emits light at the far-red end of the color spectrum, according to the team behind the work.
“There’s a real need to monitor multiple relevant molecules, as they’re doing here,” says Nicolas Tritsch, assistant professor of neuroscience at McGill University, who was not involved in the study. Because dopamine is involved in a range of key brain functions, when studying its effects on a cell it’s important to consider other neuromodulators that are released at the same time, as well as the signaling cascades these molecules may trigger, Tritsch says.
Most dopamine-tracking strategies genetically encode a naturally occurring fluorescent protein into dopamine receptors; when dopamine attaches to the modified receptors, the fluorescent protein changes shape and emits light. But naturally occurring fluorescent proteins have a limited color palette, which has made it difficult to develop sensors that can go beyond two-color imaging, says study investigator Yulong Li, professor of life sciences at Peking University.
Instead of genetically encoding a fluorescent protein, HaloDA1.0 attaches a synthetic molecule called HaloTag to dopamine receptors. This tag binds tightly to previously developed artificial dyes that change shape and fluoresce in the far-red spectrum when dopamine binds to its receptors. Because the dyes fluoresce at the far end of the red spectrum, it leaves room for other sensors to glow at different wavelengths.
Other scientists have previously combined HaloTag and synthetic dyes to create sensors, but this is the first time HaloTag has been applied to a member of the family of G protein-coupled receptors (GPCRs)—namely, dopamine receptors. “It opens up so many new possibilities, because there are so many GPCRs” that play critical roles in brain function, says Robert Campbell, professor of chemistry at the University of Tokyo, who was not involved in this study.
L
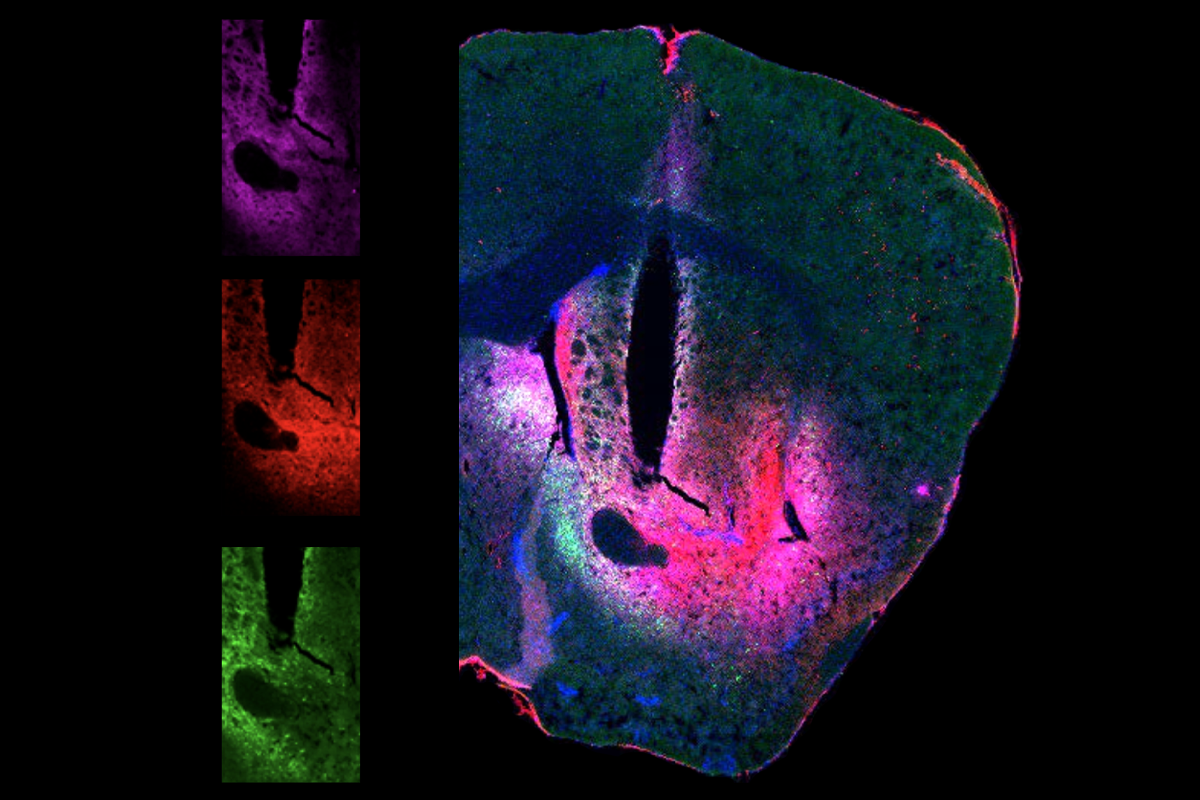
i’s team tested the sensor in living zebrafish and mice. The researchers injected a plasmid carrying the sensor into fish larvae and then submerged the fish in the dye; for the mice, they injected a virus encoding the HaloDA1.0 into select areas of the brain and then injected the dye, which can cross the blood-brain barrier, into the bloodstream.In a variety of tests, the team was able use HaloDA1.0 together with existing sensors to generate three-color imaging of dopamine and two other molecules, including acetylcholine, calcium and adenosine triphosphate (ATP). In mice, the researchers combined the sensor with optogenetics to monitor dopamine and calcium release in response to the stimulation of specific brain regions.
They also used the tool to simultaneously track dopamine, acetylcholine and the secondary messenger cyclic adenosine monophosphate (cAMP) in the nucleus accumbens in response to the mice consuming sugar or experiencing mild foot shocks. The experiments revealed how these positive and negative stimuli change dopamine and acetylcholine levels—and how that, in turn, can affect to the production of cAMP. The researchers published their findings last month in Science.
A major technological advance in this study was the discovery of a dye that, compared with other dyes researchers have tested, can more readily reach the brain, Campbell says. Other groups, including his own, can now apply this dye to their own sensors, he adds.
HaloDA1.0 has some limitations, Li notes. One is that, unlike genetically encoded fluorescent proteins, which are long-lasting, the dye used in this sensor fades after a few days. Researchers who want to carry out longer experiments may need to reinject the dye periodically. Li says his group is working on finding noninvasive ways to sustainably provide enough dye for these lengthier projects.
Another limitation is that not all confocal microscopes, which are commonly used in these studies, are set up to conduct three-color imaging, Tritsch says.
At the same time, though, the ability to use synthetic dyes mean that “we’re not limited to things that our bodies can engineer,” he adds. “This is an exciting new development that I’m sure in a few years from now, we’ll look back at and think how it really opened a whole new area of imaging.”
Recommended reading
How insights from network theory can boost interdisciplinary efforts

What are the most transformative neuroscience tools and technologies developed in the past five years?
Bespoke photometry system captures variety of dopamine signals in mice
Explore more from The Transmitter
Novel neurons upend ‘yin-yang’ model of hunger, satiety in brain
Expanding set of viral tools targets almost any brain cell type