New questions around motor neurons and plasticity
A researcher’s theory hangs muscle degeneration on a broken neural circuit.
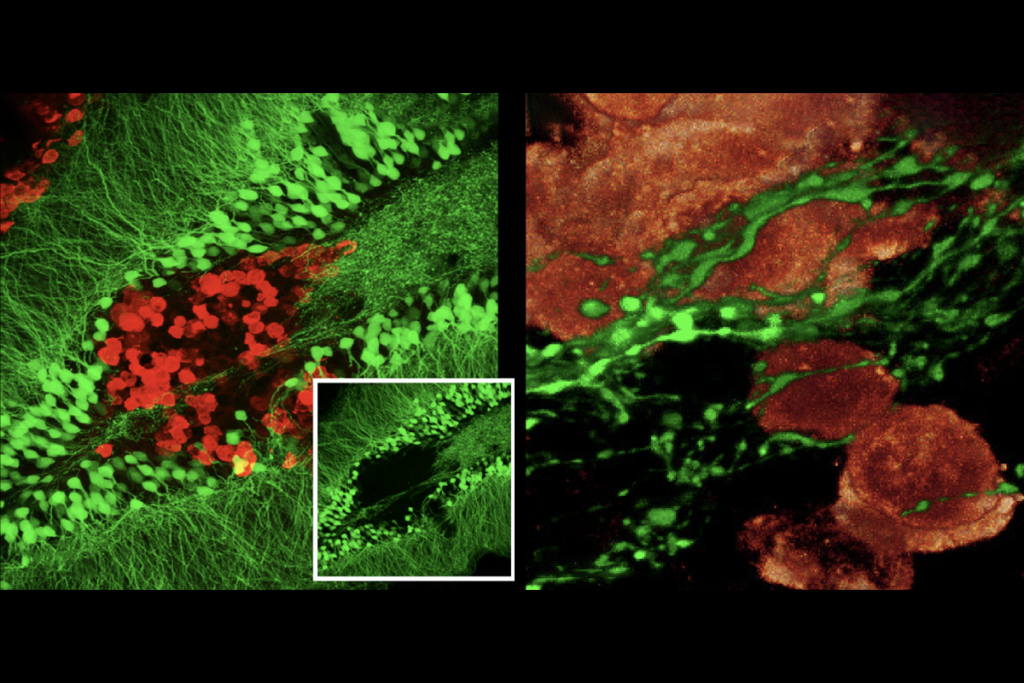
In February of this year, George Mentis and his colleagues published data from a small clinical trial they said showed that degraded motor neurons aren’t irreparable. In the study, electrical stimulation to the spine in three people with spinal muscular atrophy (SMA) appeared to resuscitate lost motor neurons, the authors said, as well as restore some of the cellular processes needed to activate muscle.
“It was incredible,” says Mentis, professor of pathology and cell biology (in neurology) at Columbia University. “We’re unleashing or tapping on the potential of dysfunctional neurons to show plasticity.”
The authors wrote that the results showed it was possible to “effectively rescue motor neuron function” and that the electrical stimulation had rebuilt neuronal circuitry and reversed—at least for a while—some degeneration.
Mentis and his team think their results are coalescing into a theory, even if they don’t fully understand it yet. The researchers are essentially altering the electrical properties of the motor neurons so they start to behave better and closer to normal, says Genís Prat-Ortega, a postdoctoral associate in the Rehab Neural Engineering Labs at the University of Pittsburgh and an investigator on the study. “The motor neurons change and repair,” he says. “Somehow, we are reversing a neurodegenerative process.”
Not everyone is so sure. Tim Hagenacker, professor of neurology at the University of Duisburg-Essen, says rebuilding the neural circuit is “not entirely convincing” as an explanation for the study’s results. He thinks that “other cell types play a crucial co-role” in restoring neuronal plasticity or that dysfunctional motor neurons could exist in some form of hibernation.
There were detractors back in 2017, too, when Mentis published a study investigating this idea in mice. “Everybody said, ‘This is not real,’” says Christian Simon, group leader at the Carl-Ludwig-Institute for Physiology at Leipzig University, who has also studied how circuitry gone awry can affect motor neurons. “The field was not ready.”
Mentis has been trying to prove it was real ever since.
S
MA is caused by a corrupt version of the SMN1 gene, which results in lower SMN1 protein production. Motor neurons cannot pass signals to muscles without SMN1, and it’s thought that the motor neurons then degenerate and die. The loss of the protein also impairs the growth of axons and dendrites.In their 2017 study, Mentis and his colleagues examined synapses between proprioceptive cells and the dendrites of motor neurons in a mouse model of SMA. The lack of SMN1 protein was associated with reduced synaptic transmission of glutamate and a decrease in motor neuron firing, they found. The results also showed that motor neuron dysfunction and death were independent events, and Mentis hypothesized that they were governed by different mechanisms.
To confirm this, the team increased the expression of SMN1 in the motor neurons, and then also in the proprioceptive neurons. Adding the protein only to motor neurons saw fewer of them die. By contrast, adding SMN1 to the proprioceptive neurons did not rescue the motor neurons they connected to, indicating that a lack of SMN1 protein in those proprioceptive neurons had not induced motor neuron death. Instead, the motor neurons were dysfunctional because of a problem in the wider circuitry, Mentis says.
In the 2023 SMA study, after stimulating the proprioceptive nerve in the three participants for two hours a day for four weeks, muscle strength improved by up to 180 percent. This benefit persisted for several weeks, suggesting enduring changes in motor neuron behavior, the authors wrote. The participants showed reversal of some motor neuron dysfunction, and inspection of knee muscle activity suggested the cells became less excitable and increased their firing rates.
But after weeks of daily stimulation, the participants also showed unusually high peak firing rates in some motor neurons and a firing-rate decay over time that looked similar to that of healthy people. The researchers called these motor neurons “rescued.”
With the recent results in SMA patients, Mentis is challenging “the dogma” around motor neurons in muscle-wasting diseases, says Rashmi Kothary, professor of medicine at the Ottawa Hospital Research Institute who develops mouse models of SMA.
Hazel Allardyce, a postdoctoral research fellow at the University of Aberdeen, studies how SMA weakens blood vessels. She says the belief that SMA is caused by a lack of SMN1 protein, which then kills motor neurons, has always been stronger in the United States than in Europe, but data emerged about a decade ago suggesting that SMN1 depletion also damages other cell and tissue types.
“If I had gone and presented my work on blood vessels in the U.S. eight years ago,” she says, “I would probably have been really toughly questioned off the stage.” But when she presented earlier this year, “it was actually very well accepted.”
Still, Mentis and Simon both say their work, when presented at conferences, has drawn skeptics. Even Doug McCullough, a 57-year-old retiree from New Jersey with SMA who participated in the 2023 study, was initially doubtful of what he was feeling. He wondered, “Am I just having a good day?” he recalls. “With a body that’s somewhat compromised, things like the temperature or if you got a good night’s sleep can make a big difference.”
Arthur Burghes, professor of molecular and cellular biochemistry at Ohio State University who has worked on the pathology of SMA since the 1990s, also has questions about the results. He says the researchers “overexaggerate the proprioceptive defect.” Although SMA mice models do show lower loss of motor neurons, that’s not the same in people, he points out. And though postmortem examination of the most severe SMA cases demonstrates “complete loss of virtually all the motor neurons,” the sensory neurons—housed in the dorsal root ganglia (DRG)—are not as severely affected.
“This makes one think that the motor neuron is the first to go and drive [the pathology], as opposed to the DRG,” Burghes says. Less severe cases of SMA also show “clear motor neuron loss, but the degree of change in sensory neurons also appears less,” he adds.
Mentis says that anatomical postmortem analysis cannot reliably judge the level of dysfunction of remaining sensory neurons. And he points out that his data show that significant numbers of motor neurons survive even in the most severe SMA cases.
Charlotte Sumner, professor of neurology and neuroscience at Johns Hopkins University, says the sticking point for some scientists is “not the idea that motor neurons are dysfunctional—rather that the dysfunction is driven by an abnormality of the sensory neurons.” And, she says, “patients don’t manifest any overt sensory abnormalities, so it’s a little bit hard to wrap your head around the idea that there’s a problem with the sensory neurons.”
I
n February, Simon and a team that included Mentis published a study showing that people with SMA who are blindfolded struggle to judge the position of their legs in space, and H-reflex scores suggested their proprioceptive neurons are affected to a greater extent than their motor neurons.Poor H-reflex scores have been recorded in people with SMA in the past, but this was attributed to the motor neurons being “so bad you can’t measure it anymore, rather than the other way around,” Simon says.
Prat-Ortega says the results support the idea that motor neuron dysfunction and death are independent events in SMA. “You can solve the dysfunction without stopping the motor neurons dying,” he says. “Or you can prevent the death of neurons, but then you don’t solve the motor neuron dysfunction.”
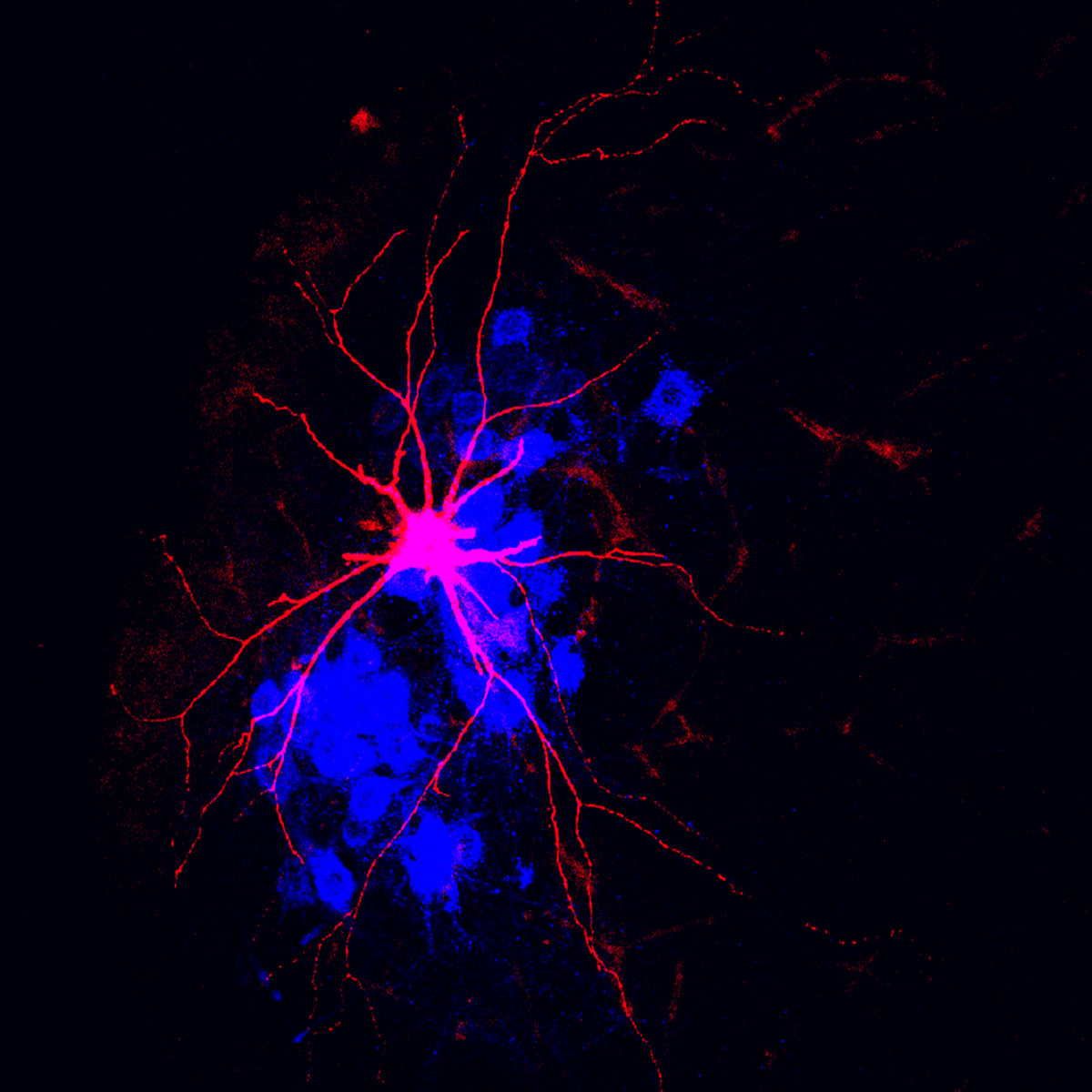
Mentis says he can’t fully explain this yet, but he thinks reduced glutamate transmission in the proprioceptive neurons decreases the synaptic input to motor neuron dendrites. The reduced input triggers maladaptive changes, such as the motor neurons expressing fewer potassium ion channels, and thus they cannot repolarize after an action potential. The motor neurons fire less and can’t reliably send signals to the muscles, which in turn atrophy. Paradoxically, the dysfunction also makes the motor neurons hyperexcitable.
It’s difficult to pinpoint where, exactly, reduced SMN1 protein fits into the circuit. But Mentis says it might involve two still-unnamed enzymes downstream of the glutamate release from the proprioceptive synapses. His team is investigating and hopes to publish soon, he says.
Simon speculates the new electrical activity in these historically dysfunctional neural circuits could encourage synapses to regrow and proliferate, forming stronger and more enduring connections to both receive signals from sensory neurons and send signals to activate muscles. But this can’t be studied in people, so Mentis says he and his team will probably go back into mice to investigate.
Grégoire Courtine, professor of neurosciences at the Swiss Federal Institute of Technology, is also looking at this area. A 2018 study from his lab showed that stimulation of proprioceptive neurons in the spinal column can help paralyzed people walk again. Earlier this year, the same team used robotic rehabilitation devices to restore control over previously paralyzed muscles even when the current was off, awakening the “dormant neuromuscular system.”
M
entis says he wants to test longer stimulation periods on SMA volunteers with severe symptoms and gauge motor neuron function restoration. He also wants to test electrical stimulation on mice treated with SMA drugs. Epidural stimulation, he notes, has also helped people with spinal cord injuries, and “we think that the mechanism might be similar, if not identical, to the motor neuron dysfunction that we have identified in SMA.”He says the same principle could even apply to broken connections between motor neurons in the brain, possibly helping those with dementia and Parkinson’s disease.
Rob Brownstone, professor of neurosurgery at University College London and an expert on neural circuits, welcomes the focus on the inner workings of the spinal cord, including the roles of the proprioceptive sensory nerves, which he says are too often overlooked. And he says it’s plausible that electrical stimulation of these sensory nerves could trigger changes to motor neuron function there. But it’s not clear if similar circuit dysfunction could play a role in other conditions, he cautions, which makes it difficult to apply the results beyond SMA.
“I would say that there would be a danger in jumping from here to other diseases that affect motor neurons,” he says.
Recommended reading

A brief history of precision self-scanning

The state of neuroscience in 2025: An overview
Explore more from The Transmitter
Worms help untangle brain structure/function mystery