Neurons fuel lung tumors that have spread to brain
Functional synapses between brain cells and cancer cells are key to the metastatic growth, according to new findings from two independent teams.
Neurons in the brain form functional connections with small-cell lung cancer cells that have spread there, fueling the growth of these brain metastases. Multiple experiments by two independent teams of researchers underpin the new finding.
The development adds to mounting evidence of a role for the nervous system in cancer initiation and progression. Neurons form synapses with brain cancer cells, past studies show, and the nervous system is also known to regulate the growth of many cancers outside the central nervous system.
But the new work provides the first definitive demonstration of functional synapses between neurons and cancer cells that originate outside the central nervous system. “It really pushes cancer neuroscience to the next level,” says Humsa Venkatesh, assistant professor of neurology at Harvard Medical School and an investigator on one of the studies.
The studies, both published 10 September in Nature, each involve dozens of interdisciplinary researchers—one group, which includes Venkatesh, is based in the U.S. and the other in Germany. Together, they marshal evidence from gene expression, electron microscopy, cell culture, electrophysiology and optogenetics, and draw on a variety of other experiments as well.
“I loved how they used some similar techniques, but other very different techniques, and came to the very same conclusion,” says Paola Vermeer, associate professor of surgery at the University of South Dakota, who was not involved in either effort. “It’s just a tour de force on both sides.”
S
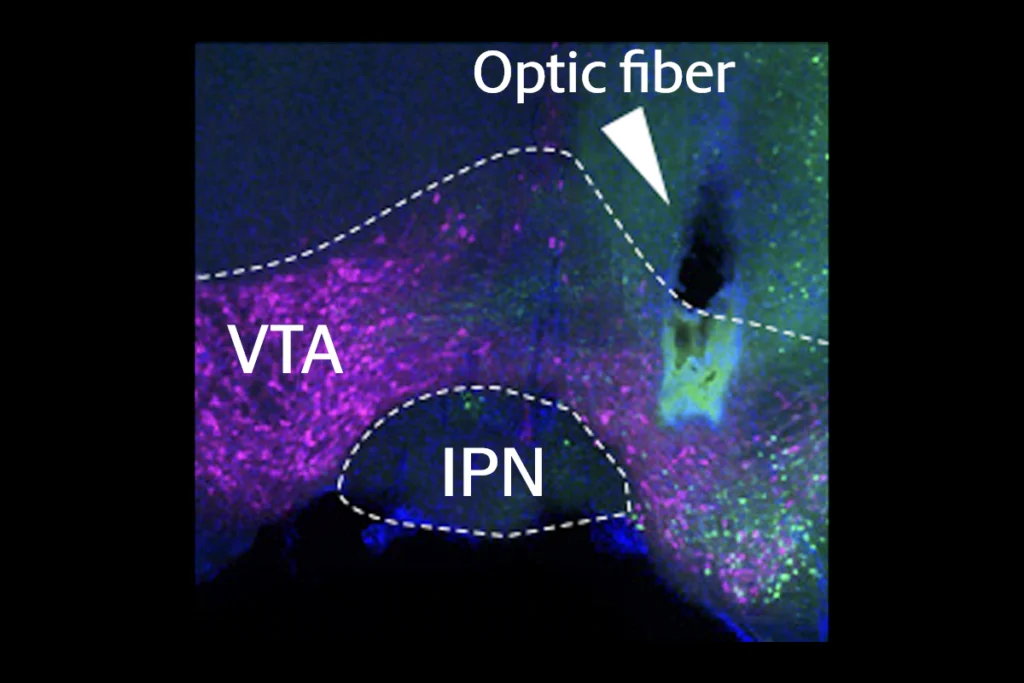
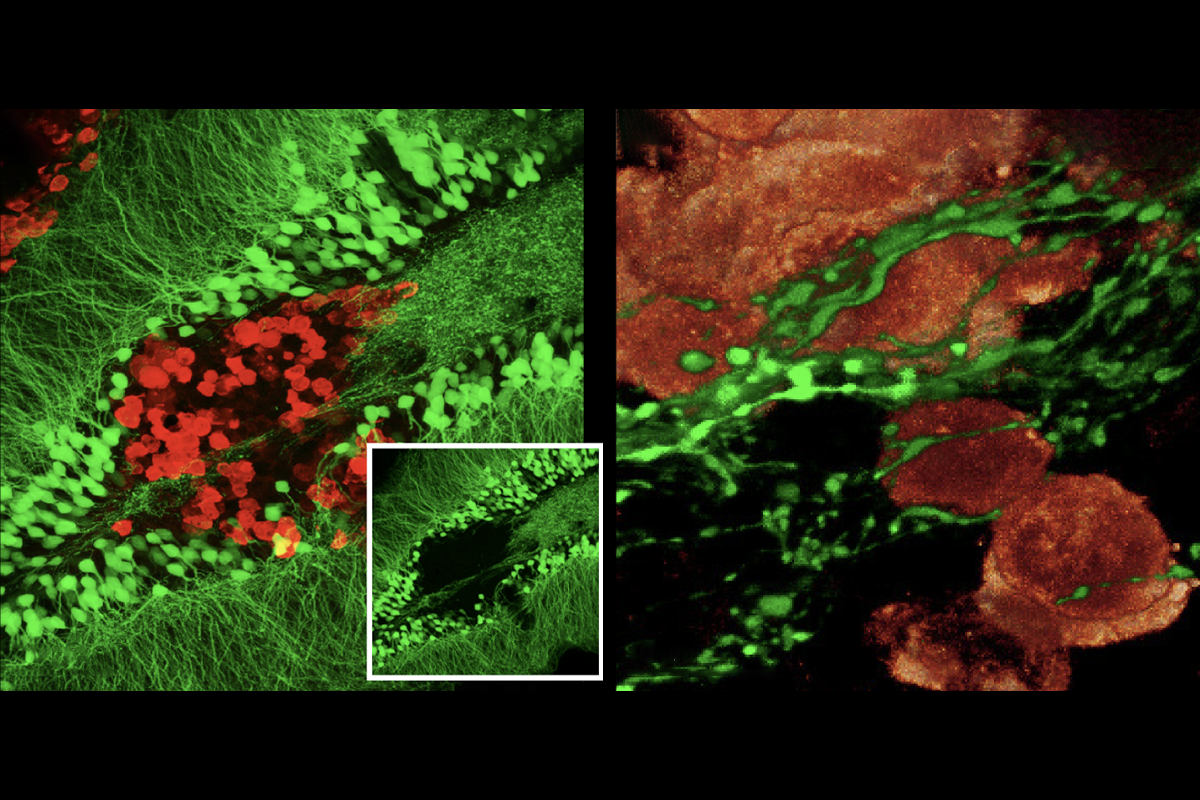
mall-cell lung cancer often spreads to the brain, and tissue samples from people with brain metastases show axons in and around these tumors. A similar pattern of axon growth occurs in the brains of mice injected with small-cell lung cancer cells that form tumors there, both teams showed.Viewed under powerful microscopes at nanoscale resolution, the contacts between the nerve cells and cancer cells in the mouse brains look like typical synapses between neurons, both teams observed.
And the synapses are functional, electrical current recordings from individual cancer cells in brain slices showed—providing compelling evidence of synaptic transmission. In addition, a fluorescent dye moved from cancer cell to neuron in the brain, according to viral tracing studies by the German team.
That team has also documented synaptic structures in primary lung tumors in mice and in laboratory dishes where neurons are cultured alongside small-cell lung cancer cells. The lab-dish synapses are functional, but it is not yet certain whether the lung tumor synapses are.
“We’re working on that,” says study investigator Filippo Beleggia, a cancer geneticist at the University of Cologne. “That’s very high on our [list of] priorities.”
Neuronal input to small-cell lung cancer cells fuels their growth, according to lab-dish experiments in both papers. Multiple cancer cell lines proliferate more in the presence of neurons than they do on their own.
In addition, synapse-formation genes are upregulated in cancer cells grown in the presence of neurons, and neurons have more synapses and show increased activity in the presence of cancer cells, the U.S. team showed, offering this and other evidence that metastatic cells adapt to the brain’s environment.
Chemicals that block nerve signals dampen cancer cell growth but do not extinguish it entirely, according to experiments from both teams. This indicates that paracrine signaling, which involves chemicals released into the extracellular space, contributes to neurons’ growth-promoting effects but that synaptic transmission plays the primary role.
“Really that direct contact was needed,” Vermeer says. “So it really shows that it is a very intimate connection that is happening between the small-cell [lung] cancer tumor cells and the neurons.”
A
ctivating neurons in the premotor cortex in mice via optogenetics triggers increased proliferation of small-cell lung cancer cells there, the U.S. team further reported, showing that the neuronal signaling also promotes tumor growth in vivo.Conversely, withdrawing nerve signals may interrupt cancer growth. When the team severed the vagus nerve in a mouse model of small-cell lung cancer before the animals had developed tumors, the mice ultimately developed fewer and smaller lung lesions. Spread to the liver was delayed or averted altogether, and the mice survived longer than animals with an intact vagus nerve.
Neuronal activity involving either the excitatory neurotransmitter glutamate or the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) can drive the proliferation of small-cell lung cancer cells, both teams found. Previous work focused mostly on excitatory transmission, but GABA has been shown to contribute to the growth of certain forms of pediatric brain cancer, says U.S. study investigator Michelle Monje, professor of pediatric neuro-oncology at Stanford University.
Because small-cell lung cancer is thought to arise from pulmonary neuroendocrine cells, which act as environmental sensors and communicate with pulmonary nerves, it is “a good place to start” looking for synaptic connections with neurons, Venkatesh says. But functional excitatory synapses also exist in brain metastases of breast and skin cancer, a third team of researchers reported in a preprint posted on bioRxiv last year. (One of that preprint’s investigators, Frank Winkler, shared this year’s Brain Prize with Monje for their discoveries in cancer neuroscience.)
“There’s a lot of lessons to be learned from the neuroscience of primary brain cancers to be applied to what we see in brain metastases,” Monje says.
U
nlike Monje and Venkatesh, Beleggia is new to cancer neuroscience. His work began with a series of bioinformatics studies, including an unbiased, across-the-genome search showing that disrupting synaptic genes promotes small-cell lung cancer in a mouse model, and a search that turned up pathogenic variants in such genes in human tumor samples.“We were not looking for this,” he says, citing that fact as a strength of the larger study that resulted. “The data kind of slapped us in the face with it.”
Beyond basic discovery, the new findings “open a completely new avenue for treatment” of small-cell lung cancer and perhaps other cancers, says Sebastien Talbot, associate professor of biomedical and molecular sciences at Queens University in Ontario, Canada, who was not involved in either study.
In fact, a widely available anti-seizure drug that indirectly both enhances inhibitory signaling and diminishes excitatory signaling dampens the growth of small-cell lung cancer tumors in the brains of mice, according to data in the U.S. team’s paper. In the other analysis, two drugs (one experimental, the other in clinical use since the 1990s) that target a type of glutamate receptor showed some, albeit less dramatic, effects on lung tumors and survival in mice.
Repurposing drugs already approved in psychiatry and neurology as part of cancer treatment “would potentially allow a very quick translation” to the clinic, Talbot says.
Recommended reading

Oxytocin prompts prairie voles to oust outsiders, fortifying their friendships
Some dopamine neurons signal default behaviors to reinforce habits

Faked results lead to retraction of high-profile cancer neuroscience study
Explore more from The Transmitter
Astrocyte networks span large swaths of brain