‘Understudied secret’ in brain dampens nicotine drive in mice
The interpeduncular nucleus produces an aversion to nicotine, even at low doses, and helps moderate how rewarding mice find the drug.
Addiction may be known as a disease of “more,” but drug-taking also taps a powerful drive for less that can suppress reward in the brain, even at low doses, according to a new study of nicotine responses in mice. The results suggest that the systems of reward and aversion that regulate addiction are more intertwined than previously thought.
“That’s absolutely fascinating, because the field has been dominated by this notion of the go, the drive to get drug, but the drive is moderated by the stop,” says Paul Kenny, professor of neuroscience at the Icahn School of Medicine at Mount Sinai, who was not involved in the work. A faulty “stop” signal could be one of the culprits in addiction, he adds.
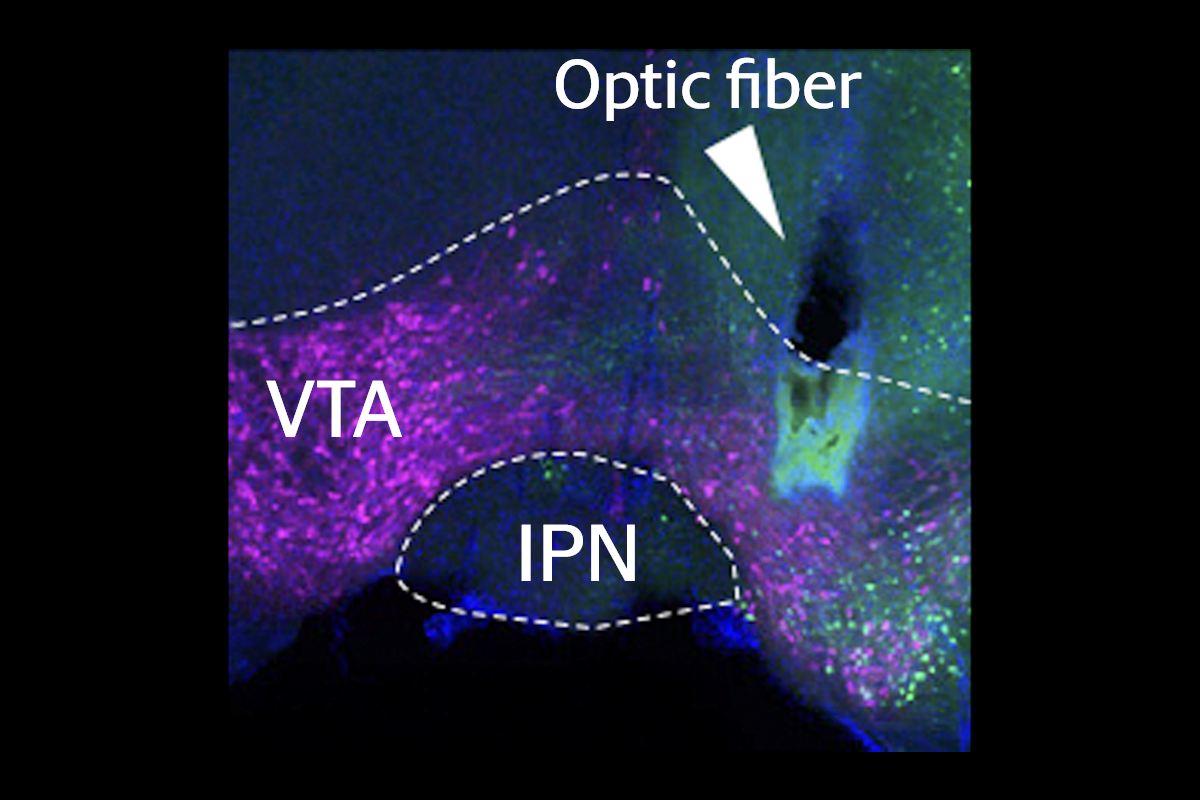
Recent studies have begun to explore this stop signal. Intravenous nicotine activates nicotinic acetylcholine receptors on dopamine neurons in the midbrain’s ventral tegmental area (VTA), generating a rewarding effect that promotes more drug consumption. And high doses activate a tiny adjacent area, the interpeduncular nucleus (IPN), which drives aversion, previous studies have suggested.
But doses too low to excite the VTA also activate the IPN in mice, the new work shows. In another experiment, the team used fluorescent proteins to find where axons from the IPN terminate and to identify the intermediate player connecting the IPN and the VTA: the laterodorsal tegmental nucleus (LDTg). The findings were published in Neuron in April.
“This was very thrilling,” says the study’s principal investigator, Alexandre Mourot, research director in brain plasticity at the Institut National de la Santé et de la Recherche Médicale (INSERM). It suggests that at very low doses, the VTA does not respond because the IPN “erases the rewarding properties of the drug,” he says.
T
o test the IPN brakes, the team devised a chemogenetic method to engineer mice in which they could permanently block a type of IPN nicotinic receptors. These mice showed 80 percent less IPN activation in response to nicotine compared with controls.But the engineered animals also showed a preference for lingering in an area of their cage where they had previously received low doses of the drug, suggesting that such doses still felt rewarding and activated the VTA. In line with this idea, the VTA lit up in the model animals at such low doses, unlike in controls, and the region showed a greater response to higher doses of nicotine.
“I was not thinking it would be strong to the point that we would see it,” Mourot says of the effect on the VTA. “That was very unexpected to me.”
The findings suggest that neuroscientists need to rethink how they understand reward and aversion, Mourot says. “It was thought to be two parallel, unrelated pathways,” he says. “What we found is that the aversion signal can decrease the reward directly, on the reward center.”
Recent studies have been leading the field to this conclusion, Kenny says. Other work has linked the IPN and neighboring medial habenula to opioid dependence, and it’s likely involved in other addictions as well, he says. “I think that this site is likely playing an important role [in addiction], at least a much more important role than is currently recognized by the field.”
An important question to resolve is how the IPN’s function could drive addiction over time, by studying how animals become dependent on nicotine, says Christian Peters, assistant professor of anatomy and cell biology at the University of Illinois Chicago, who was not involved in the new work. “It’s a harder question to ask, but I think this is a nice step.”
Meanwhile, the IPN remains an “understudied secret in the brain,” Mourot says, with its endogenous properties still largely a mystery. He says his team plans to study how the IPN might modulate reward in general, even outside of addiction.
Chemogenetic tools such as the one Mourot’s team developed will certainly help, Kenny says, noting that it’s “the beauty of this study, the real elegance.” The method made it possible to link specific receptor function to circuit changes, “getting at the heart of how nicotine is acting.”
Recommended reading

Neuroscientists need to do better at explaining basic mental health research
Cocaine, morphine commandeer neurons normally activated by food, water in mice
New autism committee positions itself as science-backed alternative to government group
Explore more from The Transmitter
Novel neurons upend ‘yin-yang’ model of hunger, satiety in brain